Purpose
The Fugl-Meyer Assessment (FMA) is a stroke-specific, performance-based impairment index. It is designed to assess motor functioning, sensation, balance, joint range of motion and joint pain in patients with post-stroke hemiplegia (Fugl-Meyer, Jaasko, Leyman, Olsson, & Steglind, 1975; Gladstone, Danells, & Black, 2002). It is applied clinically and in research to determine disease severity, describe motor recovery, and to plan and assess treatment.
In-Depth Review
Items:
The scale is comprised of five domains and there are 155 items in total:
- Motor function (in the upper and lower extremities)
- Sensation (evaluates light touch on two surfaces of the arm and leg, and position sense for 8 joints)
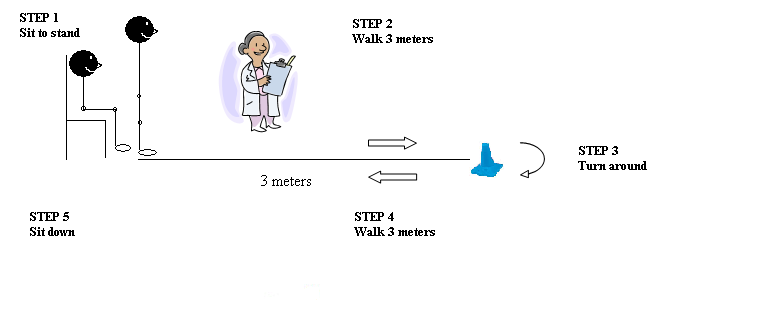
- Balance (contains 7 tests, 3 seated and 4 standing)
- Joint range of motion (8 joints)
- Joint pain
The motor domain includes items assessing movement, coordination, and reflex action of the shoulder, elbow, forearm, wrist, hand, hip, knee, and ankle. Items in the motor domain have been derived from Twitchell’s 1951 description of the natural history of motor recovery following stroke and integrates Brunnstrom’s stages of motor recovery (Gladstone et al. 2002; Poole & Whitney, 2001). Items of the FMA are intended to assess recovery within the context of the motor system. Functional tasks are not incorporated into the evaluation (Chae, Labatia, & Yang, 2003).
Time:
Sections of the FMA are often administered separately, however it takes approximately 30-35 minutes to administer the total FMA (Poole & Whitney, 2001). The average length of time for FMA administration of the Motor function, Sensation and Balance subscores have reported to range from 34 to 110 minutes, with a mean administration time of 58 minutes (Malouin, Pichard, Bonneau, Durand & Corriveau, 1994). When the motor scale is administered on its own, it takes approximately 20 minutes to complete.
A major criticism of the FMA is that it is a lengthy measure to administer (Gladstone et al., 2002). Sometimes it takes longer than 35 minutes to complete, such as when it is administered to aphasic or severely affected patients (Kusoffsky, Wadell, & Nilsson, 1982; Dettmann, Linder, & Sepic, 1987).
Scoring:
Scoring is based on direct observation of performance. Scale items are scored on the basis of ability to complete the item using a 3-point ordinal scale where 0=cannot perform; 1=performs partially; and 2=performs fully. The total possible scale score is 226.
Points are divided among the domains as follows:
- Motor function score: ranges from 0 (hemiplegia) to 100 points (normal motor performance). Divided into 66 points for upper extremity and 34 points for the lower extremity.
- Sensation score: ranges from 0 to 24 points. Divided into 8 points for light touch and 16 points for position sense.
- Balance score: ranges from 0 to 14 points. Divided into 6 points for sitting and 8 points for standing.
- Joint range of motion score: ranges from 0 to 44 points.
- Joint pain score: ranges from 0 to 44 points.
Classifications for impairment severity have been proposed based on FMA Total motor scores (out of 100 points):
Source: Finch, Brooks, Stratford, & Mayo, 2002
| Fugl-Meyer (1980) |
Fugl-Meyer et al. (1975) |
Duncan, Goldstein, Horner, Landsman, Samsa, & Matchar (1994) |
| < 50 = Severe |
|
0-35 = Very Severe |
| 50-84 = Marked |
≤ 84 = HemiplegiaComplete paralysis of the arm, leg, and trunk on one side of the body that results from damage to the parts of the brain that control muscle movements. Hemiplegia is not a progressive condition, nor is it a disease. |
36-55 = Severe |
| 85-94 = Moderate |
85-95 = Hemiparesis |
56-79 = Moderate |
| 95-99 = Slight |
96-99 = Slight motor dyscoordination |
> 79 = Mild |
Each of the five FMA domains can be separated to test a specific construct. For example, to assess upper extremity function, the subsections specifically dealing with upper extremity movement, sensation, joint motion and pain can be examined without administering the rest of the scale. Scoring of the FMA will depend on the number of items included in the subsection selected for testing.
Crow et al. (2008) proposed a shortened method of administration for the upper and lower extremity portions of the FMA. Using Guttman analysis the authors determined that scale items in the upper and lower limb sections fulfill the criteria for a valid hierarchy. Clinically this means that rather than administering the entire test, a clinician may choose to begin administering at a point in the scale that appears appropriate to the observed level of patient recovery. If a patient is able to accomplish all of the remaining scale items in the section, they are awarded a full score for that section. Likewise, when the individual being tested is unable to accomplish all the scale items in a given section, a score of 0 is given for any remaining untested, more advanced, items. This method of assessment reduces the time required to perform the test. Full guidelines for hierarchical testing procedures are provided by Crow et al. (2008)
Equipment:
The FMA requires a mat or bed, a few small objects and several different tools for the assessment of sensation, reflexes, and range of motion:
Materials needed (Poole & Whitney, 2001; Sullivan et al., 2011):
- Scrap of paper
- Ball
- Cotton ball
- Pencil
- Reflex hammer
- Cylinder (small can or jar)
- Goniometer
- Stopwatch
- Blindfold
- Chair
- Bedside table
Subscales:
There are five domains that can be assessed independently: Motor function; Sensation; Balance; Joint range of motion; and Joint pain. Sensation and Joint pain are more subjective in nature and are used less frequently (Gladstone et al., 2002). Sullivan et al. (2011) published a FMA manual of procedures, which includes training procedures for clinical practice and research trials, in an effort to standardize assessment procedures.
Training:
The FMA should be administered by a trained physical therapistIn charge of the "assessment and treatment of motor functioning, including motor control, strength and physical conditioning; balance, gait and mobility retraining; home and community visits; patient and family education regarding mobility and safety issues." (Suggested by Philips et al, 2002)
, occupational therapistIn charge of the "assessment of personal and domestic care activities; evaluation and treatment of functional impairments related to change in sensorimotor, cognitive and perceptual abilities; prescription of wheelchairs and bathroom appliances; home visits; patient and family education."(Suggested by Philips et al, 2002)
or other rehabilitation professional on a one-to-one basis with the patient (Gladstone et al., 2002).
Guidelines provided by Fugl-Meyer et al. (1975) suggest that the client should be instructed verbally and/or with a demonstration of the test. The evaluator is permitted to assist the patient in the testing of the wrist and hand to stabilize the arm (Fugl-Meyer et al., 1975). In patients confined to their beds, the joint range of shoulder abduction should be performed only to 90 degrees and extension of the hip to 0 degrees.
Alternative Forms of Fugl-Meyer Assessment (FMA)
In 1975, Fugl-Meyer, Jaasko, Leyman, Olsson, and Steglind published the FMA.
Revision to balance subscore
Subsequent to problems reported with sitting balance items (Malouin et al., 1994), Hseuh et al. (2001 as reported in Mao, Hsueh, Tang, Sheu, & Hsieh, 2002) proposed slight modifications to the scoring of the two problematic reaction items. In this modified version, patients receive a score of 0 if they lose balance easily, 1 if they partially lose balance, and 2 if they maintain sitting balance well when firmly pushed on the affected or non-affected side. The validity of the modified FMA-Balance was found to be excellent (r = 0.84).
12-item short form
Hseih et al. (2007) developed a 12-item short form of the FMA based on the upper and lower extremity domains of the FMA. Items were retained on the basis of representativeness of Brunnstrom staging and item difficulty assessed via Rasch analysis.
Client suitability
Can be used with:
- Acute and chronic patients post-stroke in settings from an acute care hospital (Wood-Dauphinee, Williams, & Shapiro, 1990) to the community (Nadeau, Arsenault, Gravel & Bourbonnais, 1999).
- Although it takes longer to administer, the FMA can be applied to severely affected patients or patients with aphasia.
Should not be used in:
- Patients who need a proxy to complete. As with other impairment indices, the FMA is scored by direct observation and therefore it cannot be used with proxy respondents.
- The FMA should not be used to detect fine or complex movements or coordination, as it measures gross limb movement only. A scale that employs a finer evaluation of isolated movements and the complete range of motor function of the upper limb only is the Motor Status Score. This scale has been found to be a reliable and valid assessment of upper limb impairment and disability following strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. (Ferraro et al., 2002).
- As an assessment of motor recovery within the context of the motor system, the FMA may separate motor recovery from functional recovery. Therefore, the FMA may not be responsive to functional improvements in chronic populations (van der Lee et al., 2001). In these instances, a more appropriate tool for assessing functional improvements in chronic populations is the Action Research Arm Test (assesses upper extremity function only).
In what languages is the measure available?
- English
- French canadian (Arsenault, Dutil, Lambert, Corriveau, Guarna, & Drouin, 1988)
Summary
| What does the tool measure? |
Motor function, sensation, balance, joint range of motion and joint pain. |
| What types of clients can the tool be used for? |
Patients with post-stroke hemiplegiaComplete paralysis of the arm, leg, and trunk on one side of the body that results from damage to the parts of the brain that control muscle movements. Hemiplegia is not a progressive condition, nor is it a disease. |
| Is this a screening or assessment tool? |
Assessment |
| Time to administer |
It takes approximately 30-35 minutes to administer the total FMA. Administration of the motor, sensation and balance subscores range from 34 to 110 minutes, with a mean administration time of 58 minutes. When the motor scale is administered on its own, it takes approximately 20 minutes to complete. |
| Versions |
- Modified FMA-Balance subscore
- 12-item short form
|
| Other Languages |
Translated and validated in French |
| Measurement Properties |
| Reliability |
Internal consistency:
Out of three studies examining internal consistency, all three reported excellent internal consistencyA method of measuring reliability . Internal consistency reflects the extent to which items of a test measure various aspects of the same characteristic and nothing else. Internal consistency coefficients can take on values from 0 to 1. Higher values represent higher levels of internal consistency..
Test-retest:
Out of six studies examining test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
, five reported excellent test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
. Two studies examined item-level agreement and found that light touch items on the FMA Sensation subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
ranged from poor to adequate; the Joint pain subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
was found to have only adequate reliabilityReliability can be defined in a variety of ways. It is generally understood to be the extent to which a measure is stable or consistent and produces similar results when administered repeatedly. A more technical definition of reliability is that it is the proportion of "true" variation in scores derived from a particular measure. The total variation in any given score may be thought of as consisting of true variation (the variation of interest) and error variation (which includes random error as well as systematic error). True variation is that variation which actually reflects differences in the construct under study, e.g., the actual severity of neurological impairment. Random error refers to "noise" in the scores due to chance factors, e.g., a loud noise distracts a patient thus affecting his performance, which, in turn, affects the score. Systematic error refers to bias that influences scores in a specific direction in a fairly consistent way, e.g., one neurologist in a group tends to rate all patients as being more disabled than do other neurologists in the group. There are many variations on the measurement of reliability including alternate-forms, internal consistency , inter-rater agreement , intra-rater agreement , and test-retest .
, however total FMA test-retest remained excellent in these studies. The study that examined longitudinal stability of the FMA items, as calculated using Rasch Analysis, reported that scores across two testing occasions are comparable.
Inter-rater:
Out of four studies examining inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
, all four reported excellent inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
(with the exception of the Balance subscore, which was found to be poor in one study). |
ValidityThe degree to which an assessment measures what it is supposed to measure.
|
Content:
Items in a modified 30-items FMA reflect the same construct, except for the item hook grasp. Based on a Guttman Scale Analysis, the motor functioning subscales can be arranged in a hierarchical sequence, allowing the use of a shortened method of administration of the FMA.
Criterion:
Predicted Motor Assessment Scale scores at 180 days after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. onset. FMA lower extremity (FMA-LE) admission subscores predicted the rehabilitation discharge Functional Independence Measure mobility and locomotion scores. FMA admission scores predicted the rehabilitation discharge Barthel Index scores. FMA-LE scores were poor predictors of mean steps per day.
Excellent correlations with Barthel Index, Motor Assessment Scale (except sitting balance items on both scales), Sensory Organization Balance Test, Action Research Arm Test, DeSouza scale, Chedoke-McMaster Stroke Assessment scale, Berg Balance Scale, Postural Assessment Scale for StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain., StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Rehabilitation Assessment of Movement (STREAM), the shortened versions of the FMA and STREAM, performance assessments of walking velocity and velocity index, and Arm Motor Ability Test.
Construct:
The FMA lower extremity subscore was able to distinguish between patients who needed assistance in walking and between three levels of self-care ability (dependent, partly dependent, and independent). Excellent correlations between the FMA and Barthel Index (except with FMA Sensastion subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
); the FMA Motor upper extremity subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
and the Action Research Arm Test; the FMA and Bobath Assessment of upper extremity; the FMA and Functional Independence Measure; the FMA Motor subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
and various measures of gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
. |
| Floor/Ceiling Effects |
A poor ceiling effectA ceiling effect occurs when test items aren't challenging enough for a group of individuals. Thus, the test score will not increase for a subsample of people who may have clinically improved because they have already reached the highest score that can be achieved on that test. In other words, because the test has a limited number of difficult items, the most highly functioning individuals will score at the highest possible score. This becomes a measurement problem when you are trying to identify changes - the person may continue to improve but the test does not capture that improvement. Example: A memory test that assesses how many words a participant can recall has a total of five words that each participant is asked to remember. Because most individuals can remember all five words, this measure has a ceiling effect. See also "floor effect." has been found with the Sensation subscore. A poor floor effectThe floor effect is when data cannot take on a value lower than some particular number. Thus, it represents a subsample for whom clinical decline may not register as a change in score, even if there is worsening of function/behavior etc. because there are no items or scaling within the test that measure decline from the lowest possible score. See also "ceiling effect."
has been found with the modified Balance subscore of the FMA at 14 days after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Another study reported an excellent floor effectThe floor effect is when data cannot take on a value lower than some particular number. Thus, it represents a subsample for whom clinical decline may not register as a change in score, even if there is worsening of function/behavior etc. because there are no items or scaling within the test that measure decline from the lowest possible score. See also "ceiling effect."
and an adequate ceiling effectA ceiling effect occurs when test items aren't challenging enough for a group of individuals. Thus, the test score will not increase for a subsample of people who may have clinically improved because they have already reached the highest score that can be achieved on that test. In other words, because the test has a limited number of difficult items, the most highly functioning individuals will score at the highest possible score. This becomes a measurement problem when you are trying to identify changes - the person may continue to improve but the test does not capture that improvement. Example: A memory test that assesses how many words a participant can recall has a total of five words that each participant is asked to remember. Because most individuals can remember all five words, this measure has a ceiling effect. See also "floor effect." for the FMA motor scores both at admission and discharge from a rehabilitation program.
|
| Does the tool detect change in patients? |
Out of 5 studies examined, 1 reported that the FMA has a large ability to detect change, 1 reported moderate, 1 reported small to moderate, and 2 reported a small ability to detect change.
|
| Acceptability |
Administration of the entire FMA is lengthy. The test is scored by direct observation and cannot be completed by proxy respondent. |
| Feasibility |
The FMA must be administered by a trained physical or occupational therapistIn charge of the "assessment of personal and domestic care activities; evaluation and treatment of functional impairments related to change in sensorimotor, cognitive and perceptual abilities; prescription of wheelchairs and bathroom appliances; home visits; patient and family education."(Suggested by Philips et al, 2002)
. It does not require any specialized equipment and can be administered across a variety of settings and has been tested for use in longitudinal assessments. |
| How to obtain the tool? |
The FMA can be obtained by following the link below (from the Institute of Rehabilitation Medicine, University of Goteberg, Goteberg, Sweden).
http://www.neurophys.gu.se/sektioner/klinisk_neurovetenskap_och_rehabilitering/neurovetenskap/rehab_med/fugl-meyer/
A version of the measure is also provided in Fugl-Meyer et al. (1975), and in the book by Dittmar, S. S. and Gresham, G. E. (1997) entitled Functional assessment and outcome measures for the rehabilitation health professional.
The FMA manual of procedures developed by Sullivan et al. (2011), can be obtained by following the link below:
http://stroke.ahajournals.org/cgi/content/full/STROKEAHA.110.592766/DC1
|
Psychometric Properties
Overview
The FMA has been used as the gold standardA measurement that is widely accepted as being the best available to measure a construct.
against which the validityThe degree to which an assessment measures what it is supposed to measure.
of other measures has been assessed. However, the reliabilityReliability can be defined in a variety of ways. It is generally understood to be the extent to which a measure is stable or consistent and produces similar results when administered repeatedly. A more technical definition of reliability is that it is the proportion of "true" variation in scores derived from a particular measure. The total variation in any given score may be thought of as consisting of true variation (the variation of interest) and error variation (which includes random error as well as systematic error). True variation is that variation which actually reflects differences in the construct under study, e.g., the actual severity of neurological impairment. Random error refers to "noise" in the scores due to chance factors, e.g., a loud noise distracts a patient thus affecting his performance, which, in turn, affects the score. Systematic error refers to bias that influences scores in a specific direction in a fairly consistent way, e.g., one neurologist in a group tends to rate all patients as being more disabled than do other neurologists in the group. There are many variations on the measurement of reliability including alternate-forms, internal consistency , inter-rater agreement , intra-rater agreement , and test-retest .
and validityThe degree to which an assessment measures what it is supposed to measure.
of the Balance subscore (the sitting balance items in particular) of the FMA has been shown to be questionable. As mentioned in the available versions section, revisions to the scoring of the Balance subscore appear to have resulted in an increase in reliabilityReliability can be defined in a variety of ways. It is generally understood to be the extent to which a measure is stable or consistent and produces similar results when administered repeatedly. A more technical definition of reliability is that it is the proportion of "true" variation in scores derived from a particular measure. The total variation in any given score may be thought of as consisting of true variation (the variation of interest) and error variation (which includes random error as well as systematic error). True variation is that variation which actually reflects differences in the construct under study, e.g., the actual severity of neurological impairment. Random error refers to "noise" in the scores due to chance factors, e.g., a loud noise distracts a patient thus affecting his performance, which, in turn, affects the score. Systematic error refers to bias that influences scores in a specific direction in a fairly consistent way, e.g., one neurologist in a group tends to rate all patients as being more disabled than do other neurologists in the group. There are many variations on the measurement of reliability including alternate-forms, internal consistency , inter-rater agreement , intra-rater agreement , and test-retest .
(Mao et al. 2002), however, further testing of the modification is required. The Sensation subscore of the FMA has also been criticized for poor face, construct and predictive validityA form of criterion validity that examines a measure's ability to predict some subsequent event. Example: can the Berg Balance Scale predict falls over the following 6 weeks? The criterion standard in this example would be whether the patient fell over the next 6 weeks.
and responsivenessThe ability of an instrument to detect clinically important change over time.
(Lin, Hsueh, Sheu, & Hsieh, 2004).
Floor/Ceiling Effects
Lin et al. (2004) examined the psychometric properties of the FMA Sensation subscore and found that these subscore had large ceiling effects at each assessment time. At 14-30 days post-stroke, 44.4% of the patients achieved the highest score, at 30-90 days, 48.9%, at 90-180 days, 62.7% and at 14-180 days, 72.1%.
Mao et al. (2002) compared the psychometric properties of the Berg Balance Scale, the modified Balance subscore of the FMA, and the Postural Assessment Scale for Stroke Patients in 123 strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. patients followed up prospectively 14, 30, 90, and 180 days after stroke onset. The modified Balance subscore of the FMA showed large floor effects (29.3%) at 14 days after stroke.
Hsueh, Hsu, Sheu, Lee, Hsieh and Lin (2009) analyzed the floor and ceiling effects for the FMA, the shortened version of the FMA, the Stroke Rehabilitation Assessment of Movement (STREAM), and the shortened version of the STREAM in 50 clients with chronic stroke. Participants were assessed at admission and discharge from a rehabilitation ward. At admission, the FMA and the shortened version of the STREAM demonstrated an excellent floor effect and ceiling effectA ceiling effect occurs when test items aren't challenging enough for a group of individuals. Thus, the test score will not increase for a subsample of people who may have clinically improved because they have already reached the highest score that can be achieved on that test. In other words, because the test has a limited number of difficult items, the most highly functioning individuals will score at the highest possible score. This becomes a measurement problem when you are trying to identify changes - the person may continue to improve but the test does not capture that improvement. Example: A memory test that assesses how many words a participant can recall has a total of five words that each participant is asked to remember. Because most individuals can remember all five words, this measure has a ceiling effect. See also "floor effect." with no participants scoring the minimum or maximum scores. The other measures showed adequate floor and ceiling effects with 2 to 18% of patients scoring the lowest or highest scores. At discharge, the FMA and the shortened version of the STREAM demonstrated an excellent floor effectThe floor effect is when data cannot take on a value lower than some particular number. Thus, it represents a subsample for whom clinical decline may not register as a change in score, even if there is worsening of function/behavior etc. because there are no items or scaling within the test that measure decline from the lowest possible score. See also "ceiling effect."
with 0% of participants scoring 0. The other measures showed adequate floor and ceiling effects, with the proportion of patients scoring the minimum and maximum scores ranging from 1 to 20%.
Reliability
Lin et al. (2004) examined the internal consistencyA method of measuring reliability . Internal consistency reflects the extent to which items of a test measure various aspects of the same characteristic and nothing else. Internal consistency coefficients can take on values from 0 to 1. Higher values represent higher levels of internal consistency. of the FMA in 176 patients with stroke from 14 to 180 days after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Cronbach’s alphas for the FMA at four time points post-stroke were excellent, ranging from alpha = 0.94 to 0.98. The inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
of the total score of the FMA was also excellent, with an intraclass correlation coefficient (ICC)Intraclass correlation (ICC) is used to measure inter-rater reliability for two or more raters. It may also be used to assess test-retest reliability. ICC may be conceptualized as the ratio of between-groups variance to total variance. of 0.93. However, item-level agreement for light touch items on the FMA Sensation subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
ranged from poor to adequate (weighted kappa ranged from 0.30 to 0.55).
Platz, Pinkowski, van Wijck, Kim, di Bella, and Johnson (2005) tested the test-retest and the inter-rater reliabilities of the FMA upper extremity items (including items from the Motor function, Sensation and passive Joint motion/Joint pain subscores), the Action Research Arm Test, and the Box and Block Test in patients with upper limb paresis either from strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. (n=37), multiple sclerosis (n=14), or from traumatic brain injury (n=5). Test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the FMA, calculated using ICC’s, was excellent (ICC = 0.97 for Total motor score; ICC = 0.81 for Sensation, ICC = 0.95 for passive Joint motion/Joint pain). Inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
for the FMA upper extremity subscore as calculated using the ICC was also excellent (ICC = 0.99 for Total motor score; ICC = 0.98 for Sensation; and ICC = 0.98 for passive Joint motion/Joint pain).
Mao et al. (2002) compared the psychometric properties of the Berg Balance Scale, the modified Balance subscore of the FMA, and the Postural Assessment Scale for Stroke Patients, in 123 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. followed up prospectively 14, 30, 90, and 180 days after stroke onset. The median of weighted kappa statistics for each item of the FMA Balance subscore was 0.79 (ranging from 0.71 to 0.95), indicating excellent individual item agreement. The ICC for the total score of the FMA Balance subscore was 0.92 (ranging from 0.88 to 0.95), indicating excellent total score agreement. The Cronbach’s alpha for the FMA Balance subscore ranged from alpha = 0.85 to 0.91 on all follow-up times, indicating high internal consistencyA method of measuring reliability . Internal consistency reflects the extent to which items of a test measure various aspects of the same characteristic and nothing else. Internal consistency coefficients can take on values from 0 to 1. Higher values represent higher levels of internal consistency..
Duncan, Propst, and Nelson (1983) examined the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
and the inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
of the FMA in 18 patients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
was examined with 5 different therapists. Pearson correlations between therapists for each component of the FMA Motor domain upper extremity subscale were found to be excellent, ranging from r = 0.96 to r = 0.97. The Motor domain lower extremity subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
correlations were also excellent, ranging from r = 0.83 to r = 0.95. The Total score correlation was high (r = 0.99). Only the reflex and coordination subscores in the upper extremity were found to be unreliable as they were significantly different between the raters across the test times.
To assess the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the FMA in Duncan et al. (1983), one therapist evaluated patients on three separate occasions at 3-week intervals. Pearson correlations were excellent for the Total FMA score (r = 0.98 to r = 0.99), Motor domain upper extremity subscore (r = 0.995 to r = 0.996), Motor domain lower extremity subscore (r = 0.96), Sensation subscore (r = 0.95 to r = 0.96), Joint range of motion/Joint pain subscore (r = 0.86 to r = 0.99) and Balance subscore (r = 0.89 to r = 0.98). No significant differences across evaluation times were found (as assessed by a repeated measures analysis of variance).
Sanford, Moreland, Swanson, Stratford, and Gowland (1993) examined the inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
of the FMA in 12 patients between 6 days to 6 months post-stroke. Patients were evaluated one day apart by three physical therapists. The inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
of the FMA was found to be excellent, with an overall ICC of 0.96 for the Total score. The ICCs for the Motor domain upper extremity subscore, Motor domain lower extremity subscore, Balance, Sensation, and Joint range of motion were excellent (0.97, 0.92, 0.93, 0.85 and 0.85, respectively). The ICC for the Joint pain subscore was the least reliable, but still adequate, with an ICC of 0.61.
Beckerman, Vogelaar, Lankhorst, and Verbeek (1996) examined the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the FMA in 49 patients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Patients were evaluated twice by one therapist, three weeks apart. The ICC for the Motor domain lower extremity subscore was excellent (ICC = 0.86), but was found to be poor for the Balance subscore (ICC = 0.34). Although the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the Balance subscore was not found to be very reliable, indicating that the patients’ performance was inconsistent, the inter-rater agreement was high.
Van der Lee, Beckerman, Lankhorst and Bouter (2001) examined the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the FMA Motor domain upper extremity subscore in 22 patients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Two baseline measurements were performed before treatment, as well as a follow-up measurement after 2 weeks. Using limits of agreement as a measure of test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
, a mean difference on test-retest within a stable population during the baseline period (14-20 days) on the Motor domain upper extremity subscore was small (0.8 points).
Woodbury, Velozo, Richards, Duncan, Studenski, and Lai (2008) investigated the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the FMA through longitudinal stability of the upper extremity items in 377 clients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. with a mean age of 60.2 years (SD 11.2). Longitudinal stability reflects the invariance of an item-difficulty hierarchy of a measure across 2 testing occasions. Assessments with a slightly modified FMA (the 3 reflex items were removed with the remaining 30 items arranged in a hierarchical sequence) were performed at admission and at 6 months post-stroke. Longitudinal equivalence of the FMA item structure was analyzed by estimating the relationship between item-difficulty calibrations at baseline and 6 months follow-up. Intraclass Correlation Coefficient (ICC)Intraclass correlation (ICC) is used to measure inter-rater reliability for two or more raters. It may also be used to assess test-retest reliability. ICC may be conceptualized as the ratio of between-groups variance to total variance. was performed and showed excellent reliabilityReliability can be defined in a variety of ways. It is generally understood to be the extent to which a measure is stable or consistent and produces similar results when administered repeatedly. A more technical definition of reliability is that it is the proportion of "true" variation in scores derived from a particular measure. The total variation in any given score may be thought of as consisting of true variation (the variation of interest) and error variation (which includes random error as well as systematic error). True variation is that variation which actually reflects differences in the construct under study, e.g., the actual severity of neurological impairment. Random error refers to "noise" in the scores due to chance factors, e.g., a loud noise distracts a patient thus affecting his performance, which, in turn, affects the score. Systematic error refers to bias that influences scores in a specific direction in a fairly consistent way, e.g., one neurologist in a group tends to rate all patients as being more disabled than do other neurologists in the group. There are many variations on the measurement of reliability including alternate-forms, internal consistency , inter-rater agreement , intra-rater agreement , and test-retest .
(ICC = 0.95). Consistency of item-difficulty calibrations across testing occasions disregarding clients’ characteristics such as age and gender, as calculated using Differential Item Functioning (DIF), showed that only two items on the FMA (1: shoulder flexion to 180° with elbow extended and 2: movement of the arm with normal speed) had a large DIF, which implied that those two items were unstable between assessments. Despite those items, the authors reported that the scores across testing occasions could still be compared as a repeat Rasch analysisRasch analysis is a statistical measurement method that allows the measurement of an attribute - such as upper limb function - independently of particular tests or indices.  It creates a linear representation using many individual items, ranked by item difficulty (e.g. picking up a very small item, versus a task requiring a very gross grasp) and person ability.   A well performing Rasch model will have items hierarchically placed from simple to more difficult, and individuals with high abilities should be able to perform all the items below a level of difficulty. The Rasch model is statistically strong because it enables ordinal measures to be converted into meaningful interval measures. It also allows information from various tests or tools with different scoring systems to be applied using the Rasch model.
with the unstable items removed found a small difference on the FMA scores (0.06 logits).
Hsueh et al. (2009) analyzed the test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
of the FMA, the STREAM, and their shortened versions in 60 clients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Participants were assessed twice within a 1 week interval. Test-retest reliabilityA way of estimating the reliability of a scale in which individuals are administered the same scale on two different occasions and then the two scores are assessed for consistency. This method of evaluating reliability is appropriate only if the phenomenon that the scale measures is known to be stable over the interval between assessments. If the phenomenon being measured fluctuates substantially over time, then the test-retest paradigm may significantly underestimate reliability. In using test-retest reliability, the investigator needs to take into account the possibility of practice effects, which can artificially inflate the estimate of reliability (National Multiple Sclerosis Society).
, as calculated using Intraclass CorrelationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
Coefficient was excellent for all four measures: FMA (ICC = 0.98), the shortened version of the FMA (ICC = 0.96), the STREAM (ICC = 0.98) and the shortened version of the STREAM (ICC = 0.97).
Sullivan et al. (2011) developed a standardized measurement method and rater training program for the FMA and then used inter-rater and intra-rater reliabilityThis is a type of reliability assessment in which the same assessment is completed by the same rater on two or more occasions. These different ratings are then compared, generally by means of correlation. Since the same individual is completing both assessments, the rater's subsequent ratings are contaminated by knowledge of earlier ratings.
to examine the effectiveness of the program. After attending the training program, 17 physiotherapists and one expert rater (with 30 years experience) evaluated 15 patients with subacute strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. The initial assessments were video-recorded and later reviewed by the expert rater. The intra-rater reliabilityThis is a type of reliability assessment in which the same assessment is completed by the same rater on two or more occasions. These different ratings are then compared, generally by means of correlation. Since the same individual is completing both assessments, the rater's subsequent ratings are contaminated by knowledge of earlier ratings.
of the expert rater, as calculated using ICC was excellent for all domains of the FMA (ranging from ICC = 0.95 to 1.0). The inter-rater reliabilityA method of measuring reliability . Inter-rater reliability determines the extent to which two or more raters obtain the same result when using the same instrument to measure a concept.
between the expert rater and trained therapists, as calculated using ICC, was also excellent for all domains of the FMA (ranging from ICC = 0.87 to 0.99).
Validity
Content:
Woodbury, Velozo, Richards, Duncan, Studenski, and Lai (2008) investigated the content validityRefers to the extent to which a measure represents all aspects of a given social concept. Example: A depression scale may lack content validity if it only assesses the affective dimension of depression but fails to take into account the behavioral dimension.
of the upper extremity items of the FMA in 377 clients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. with a mean age of 60 years (SD 11,2). Assessments with a slightly modified FMA (the 3 reflex items were removed with the remaining 30 items arranged in a hierarchical sequence) were performed at admission and at 6 months post-stroke. At the follow-up evaluation, the 30-item Fugl-Meyer Assessment, as calculated using Rasch AnalysisRasch analysis is a statistical measurement method that allows the measurement of an attribute - such as upper limb function - independently of particular tests or indices.  It creates a linear representation using many individual items, ranked by item difficulty (e.g. picking up a very small item, versus a task requiring a very gross grasp) and person ability.   A well performing Rasch model will have items hierarchically placed from simple to more difficult, and individuals with high abilities should be able to perform all the items below a level of difficulty. The Rasch model is statistically strong because it enables ordinal measures to be converted into meaningful interval measures. It also allows information from various tests or tools with different scoring systems to be applied using the Rasch model.
, showed an acceptable fit statistics, except for the item hook grasp. This result suggests that all items reflect the same construct, except for the item hook grasp.
Crow & Harmeling-van der Wel (2008) analyzed the content validityRefers to the extent to which a measure represents all aspects of a given social concept. Example: A depression scale may lack content validity if it only assesses the affective dimension of depression but fails to take into account the behavioral dimension.
of the motor functioning domain of the FMA in 62 clients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. by performing a Guttmann Scale Analysis. Each motor functioning subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
of the FMA, excluding the reflex items, was arranged in a hierarchical sequence of difficulty according to the total number of passes per item. All subscales, when analyzed separately, exceed the critical values for two indices: coefficient of reproducibility (> 0,9) and coefficient of scalability (>0,7). When analyzing across all upper and lower extremity items (ignoring the subscales) the coefficient of reproducibility (0,8) and scalability (0,6) were just below the acceptable levels. The results of this study suggest the existence of a valid, cumulative, and unidimensional Guttman scale within each motor functioning subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
. In summary, if the client succeeds in completing the most difficult item in a subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
, this suggests he/she will succeed in the easier items for that same subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
. Similarly, failure on an item suggests the client will be unable to complete the remaining more challenging items in the subscaleMany measurement instruments are multidimensional and are designed to measure more than one construct or more than one domain of a single construct. In such instances subscales can be constructed in which the various items from a scale are grouped into subscales. Although a subscale could consist of a single item, in most cases subscales consist of multiple individual items that have been combined into a composite score (National Multiple Sclerosis Society).
. Therefore, a shortened method of administration of the FMA can be used with clients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain..
Criterion:
There is a lack of information available regarding the criterion validityExamines the extent to which a measure provides results that are consistent with a gold standard . It is typically divided into concurrent validity and predictive validity .
of the FMA, as the FMA was created at a time when no other similar impairment index existed against which the FMA could be tested (Gowland, Van Hullenaar, & Torresin, 1995; Gowland, Stratford, & Ward, 1993).
Bernspang et al. (1987) used the Pearson correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficient and found that the FMA correlated excellently with self-care ability scores (r = 0.64) in 109 clients measured within 2 weeks of strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain..
Nadeau et al. (1999) identified the most important clinical variables for determining gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
speed in 16 patients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. using Pearson’s correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficients. The FMA Sensation subscore correlated poorly with both comfortable and maximal gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
speeds (r = 0.14 and r = 0.05, respectively). Patients with decreased sensation and a score below 12, combined with the strength of the hip flexors, and ankle plantar flexors predicted maximal gait speed. Excellent correlations were found between the FMA Total motor score and comfortable (mean 0.76 meters/second; r = 0.61) and maximal speeds (mean 1.09 meter/second; r = 0.61).
Concurrent:
Wood-Dauphinee et al. (1990) compared the FMA to the Barthel Index in 167 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. at two time points: the acute stage (3 to 5 days post-stroke), and 5 weeks post-stroke. Using Pearson correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficients, the correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
between the FMA Motor domain upper extremity subscore and the Barthel Index total score was excellent at both the acute stage (r = 0.75) and at 5 weeks (r = 0.82). Similarly, excellent correlations were found between the FMA Motor domain lower extremity subscore and the Barthel Index at the acute stage (r = 0.77) and at 5 weeks (r = 0.89).
Poole and Whitney (1988) examined the concurrent validityTo validate a new measure, the results of the measure are compared to the results of the gold standard obtained at approximately the same point in time (concurrently), so they both reflect the same construct. This approach is useful in situations when a new or untested tool is potentially more efficient, easier to administer, more practical, or safer than another more established method and is being proposed as an alternative instrument. See also "gold standard."
of the FMA with the Motor Assessment Scale in 30 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. High correlations were found between the total scores on the FMA and the Motor Assessment Scale (r = 0.88), and between specific item scores, except sitting balance (ranging from r = 0.28 to r = 0.92).
Malouin et al. (1994) administered the FMA and the Motor Assessment Scale to 32 patients early after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain., and reported an excellent Spearman correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
for Total FMA and Total Motor Assessment Scale scores (r = 0.96). The correlations for items from the Motor Assessment Scale and corresponding FMA items were excellent, ranging from r = 0.65 to r = 0.93. The FMA Sensation scores of light touch (r = 0.64) and position sense (r = 0.67) correlated with the Motor Assessment Scale’s Balance score, but not with FMA Sitting balance items (r = 0.12 and -0.10 respectively) suggesting that the FMA sitting balance test is not valid for measuring balance. Standing balance correlations ranged from moderately to highly correlated with both the Motor Assessment Scale and FMA light touch and position sense (ranging from r = 0.43 to r = 0.67).
Di Fabio and Badke (1990) examined standing balance and dynamic weight shifting in 10 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. using a sensory organization balance test and the FMA. The results of both clinical tests were compared to determine whether the sensory organization balance test correlated with functional ability using the Spearman rank order correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficient. Scores on the lower extremity items and balance items of the FMA correlated excellently with the Sensory Organization Balance Test (r = 0.77).
De Weerdt and Harrison (1985) compared the Motor domain upper extremity subscore of the FMA with the Action Research Arm Test. Both assessments were administered to 53 hospital inpatients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. who suffered a motor deficit. The Action Research Arm Test and the FMA were excellently correlated at both 2 weeks (r = 0.91) and at 8 weeks (r = 0.94) post-stroke.
Berglund and Fugl-Meyer (1986) compared the FMA to the DeSouza scale (another assessment of upper limb function) in 50 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. who suffered a motor deficit. Excellent correlations were found with the Upper extremity Motor scores (r = 0.90). The two tests co-varied, and explained 90% of the variation in the Total scores and 80% of the Motor scores.
Gowland et al. (1993) demonstrated the concurrent validityTo validate a new measure, the results of the measure are compared to the results of the gold standard obtained at approximately the same point in time (concurrently), so they both reflect the same construct. This approach is useful in situations when a new or untested tool is potentially more efficient, easier to administer, more practical, or safer than another more established method and is being proposed as an alternative instrument. See also "gold standard."
of the Chedoke-McMaster StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Assessment scale with the FMA. The Total score of the impairment inventory of the Chedoke-McMaster StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Assessment Scale correlated highly with that of the FMA (r = 0.95). Correlations reported between impairment subscores on the FMA and corresponding subscores from the impairment inventory of the Chedoke-McMaster StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Assessment ranged from adequate to excellent (r = 0.76 to r = 0.95).
Mao et al. (2002) compared the Berg Balance Scale, the modified Balance subscore of the FMA, and the Postural Assessment Scale for StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain., in 123 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. followed up prospectively at 14, 30, 90, and 180 days after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. onset. There was excellent concurrent validityTo validate a new measure, the results of the measure are compared to the results of the gold standard obtained at approximately the same point in time (concurrently), so they both reflect the same construct. This approach is useful in situations when a new or untested tool is potentially more efficient, easier to administer, more practical, or safer than another more established method and is being proposed as an alternative instrument. See also "gold standard."
(as measured by Spearman correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficient) between the Balance subscore of the FMA and the Berg Balance Scale and Postural Assessment Scale for StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. at all follow-up times (ranging from r = 0.90 to r = 0.97).
Kusoffsky, Wadell and Nilsson (1982) reported a relationship between sensory functioning and subsequent motor recovery, as excellent correlations between sensory evoked potentials in 16 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. was observed. The relationship between sensory evoked potentials and the FMA upper extremity subscore was the most strong, and the relationship between the FMA lower extremity subscore was less strong. The strength of the relationship endured regardless of when the FMA was administered.
Feys, Van Hees, Bruyninck, Mercelis and De Weerdt (2000) assessed the role of sensory evoked potentials and motor evoked potentials in the prediction of arm motor recovery in 64 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. with a motor deficit of the arm. Patients were followed from 2 weeks to 12 months post-stroke. In this study, a poor relationship was found between sensory evoked potentials and the FMA.
Dettmann et al. (1987) administered the FMA and a walking performance test using interrupted light photography and postural maneuvers while standing on a force platform in 15 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Correlations between the FMA Total scores and performance assessments of walking velocity (r = 0.64), cadence (r = 0.58), stride-length (r = 0.53), non-paretic stance (r = 0.59), velocity index (r = 0.67), and platform measures of upright stability (r = 0.52 to r = 0.69) ranged from adequate to excellent.
Poole and Whitney (1988) examined the concurrent validityTo validate a new measure, the results of the measure are compared to the results of the gold standard obtained at approximately the same point in time (concurrently), so they both reflect the same construct. This approach is useful in situations when a new or untested tool is potentially more efficient, easier to administer, more practical, or safer than another more established method and is being proposed as an alternative instrument. See also "gold standard."
of the Motor Assessment Scale and the FMA in 30 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Excellent correlations between FMA items and corresponding MAS items were found (ranging from r = 0.64 to r = 0.92). However, one exception was the correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
of the FMA sitting balance with the MAS balance while sitting, which were poorly correlated (r = 0.28).
Chae, Labatia, and Yang (2003) evaluated the concurrent validityTo validate a new measure, the results of the measure are compared to the results of the gold standard obtained at approximately the same point in time (concurrently), so they both reflect the same construct. This approach is useful in situations when a new or untested tool is potentially more efficient, easier to administer, more practical, or safer than another more established method and is being proposed as an alternative instrument. See also "gold standard."
of the Arm Motor Ability Test using the FMA as the criterion measure of post-stroke upper limb motor impairmentLoss of strength and coordination, decrease in arm or leg movement
in 30 patients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Excellent Spearman correlations between upper extremity scores of the FMA and Arm Motor Ability Test functional ability scores (r = 0.94) as well as between upper extremity scores of the FMA and Arm Motor Ability Test quality of movement scores (r = 0.94) were reported.
Hsueh et al. (2009) analyzed the concurrent validityTo validate a new measure, the results of the measure are compared to the results of the gold standard obtained at approximately the same point in time (concurrently), so they both reflect the same construct. This approach is useful in situations when a new or untested tool is potentially more efficient, easier to administer, more practical, or safer than another more established method and is being proposed as an alternative instrument. See also "gold standard."
of the FMA, the shortened version of the FMA, the STREAM, and the shortened version of the STREAM in 50 clients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Excellent Spearman correlations were found between all four measures (ranging from rho = 0.91 to rho = 0.99).
Predictive:
Mao et al. (2002) compared the psychometric properties of the Berg Balance Scale, the modified Balance subscore of the FMA, and the Postural Assessment Scale for StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. in 123 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. at 14, 30, 90, and 180 days after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. onset. Excellent Spearman correlations were found between the scores of the Balance subscore of the FMA at the earlier 3 time points and the Postural Assessment Scale for StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. scores (ranging from r = 0.80 to r = 0.87), indicating excellent predictive validityof the FMA modified Balance subscore.
Chae, Johnston, Kim, and Zorowitz (1995) found that FMA lower extremity admission subscores predicted the rehabilitation discharge Functional Independence Measure mobility (r = 0.63) and locomotion (r = 0.74) scores in 48 patients at 6 weeks post-stroke.
Hsueh et al. (2009) analyzed whether the motor scores of FMA, of the shortened version of the FMA, the STREAM, and the shortened version of the STREAM measured at admission to a rehabilitation program were able to predict Barthel Index scores at discharge in 50 clients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Excellent Spearman correlations were found between the Barthel Index scores and the FMA (rho = 0.72), the shortened version of the FMA (rho = 0.74), the STREAM (rho = 0.75) and the shortened version of the STREAM (rho = 0.77). These results suggest that all measures were able to predict Barthel Index scores at discharge.
Fulk, Reynolds, Mondal & Deutsch (2010) examined the predictive validityA form of criterion validity that examines a measure's ability to predict some subsequent event. Example: can the Berg Balance Scale predict falls over the following 6 weeks? The criterion standard in this example would be whether the patient fell over the next 6 weeks.
of the 6MWT and other widely used clinical measures (FMA-LE, self-selected gait-speed, SIS and BBS) in 19 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. The FMA-LE was found to be a poor predictor of mean steps per day (r = 0.06; p = 0.798). Although gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
speed and balance were related to walking activity, only the 6MWT was found to be a predictor of community ambulation in patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain..
Construct:
Several validation studies have provided good evidence that the FMA is measuring what it is intended to measure. The construct validityReflects the ability of an instrument to measure an abstract concept, or construct. For some attributes, no gold standard exists. In the absence of a gold standard , construct validation occurs, where theories about the attribute of interest are formed, and then the extent to which the measure under investigation provides results that are consistent with these theories are assessed.
of the FMA has been examined by comparing the scale with other measures of strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. recovery that reflect post-stroke independence in activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living or disability level.
Sonde, Gip, Fernaeus, Nilsson, and Viitanen (1998) found that FMA scores reflected changes in upper extremity scores after low frequency transcutaneous electric nerve stimulation. A Mann-Whitney U-test was used to test the significance of the differences in FMA scores at the start and end of the study between the treatment and control groups. This test revealed that the FMA scores differed significantly between the treatment and control groups (Mann-Whitney U = 130.5). Further, the Spearman rank correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
between the FMA scores was excellent (r = 0.95).
Chae, Bethoux, Bohine, Dobos, Davis, and Friedl (1998) assessed the efficacy of neuromuscular stimulation in enhancing the upper extremity motor and functional recovery of 28 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. FMA scores reflected changes in upper extremity scores after neuromuscular stimulation. Parametric analyses revealed significantly greater gains in FMA scores for the treatment group immediately following treatment (13.1 versus 6.5), at 4 weeks after treatment (17.9 versus 9.7), and at 12 weeks after treatment (20.6 versus 11.2). Gains were observed in FMA scores but not with Functional Independence Measure scores.
Kraft, Fitts, and Hammond (1992) tested functional improvement in the upper limb of 22 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. who received either EMG-initiated electrical stimulation of wrist extensors, low-intensity electrical stimulation of wrist extensors combined with voluntary contractions, proprioceptive neuromuscular facilitation exercises, or no treatment. During the course of treatment, FMA scores of patients receiving proprioceptive neuromuscular facilitation improved 18%, patients receiving low-intensity electrical stimulation of wrist extensors combined with voluntary contractions improved 25%, and patients receiving EMG-initiated electrical stimulation of wrist extensors improved 42%. FMA improvement of the treated groups was significant from pre-treatment to post-treatment, and the improvement was maintained at three-month and nine-month follow-up sessions. In contrast, the control group showed no significant change in FMA scores or grip strength.
Feyes, deWeerdt, Seltz, Steck, Spichinger, and Vereeck (1998) randomized 100 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. to an experimental group that received sensorimotor stimulation for 6 weeks or to a control group. Patients were evaluated before, during, and after the intervention period and at 6 and 12 months after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Only scores on the FMA showed group differences at follow-up. Scores on the Action Research Arm Test and the Barthel Index did not show group differences.
Duncan et al. (1998) randomized 20 patients with mild or moderate strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. who had completed inpatient rehabilitation to receive a home-based exercise program or usual care. The experimental group demonstrated more improvement in the FMA Motor domain upper and lower extremity subscores than did the usual care group. However, the differences in motor recovery were only significant for the Motor domain lower extremity (Motor domain upper extremity mean change in score = 8.4 versus 2.2; Motor domain lower extremity mean change in score = 4.7 versus -0.9).
Malouin et al. (1994) tested 32 patients with the FMA and the Motor Assessment Scale on two consecutive days and found that the FMA distinguished between patients with minimal recovery better than the Motor Assessment Scale. Adequate to excellent negative correlations between score differences and levels of recovery (upper extremity r = -0.50 and lower extremity r = -0.69) were found, indicating that the largest differences between the two measures were in earlier stages of recovery or among more severely affected individuals.
Platz et al. (2005) tested the construct validityReflects the ability of an instrument to measure an abstract concept, or construct. For some attributes, no gold standard exists. In the absence of a gold standard , construct validation occurs, where theories about the attribute of interest are formed, and then the extent to which the measure under investigation provides results that are consistent with these theories are assessed.
of the FMA upper extremity items (including items from the Motor function, Sensation and passive Joint motion/Joint pain subscores), the Action Research Arm Test, the Box and Block Test and the Motricity Index, using the Spearman correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficient, in patients with upper limb paresis either from strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. (n=37), multiple sclerosis (n=14) or from traumatic brain injury (n=5). Excellent correlations were found between the FMA and the Action Research Arm Test (r = 0.93), the Box and Block Test (r = 0.92), and the Motricity Index (r = 0.86). The FMA was also correlated with more general measures of impairment and activity limitation, including the Ashworth Scale, the Hemispheric StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Scale and the modified Barthel Index. An excellent correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
was found between the FMA and the Hemispheric StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Scale (r = -0.69), and an adequate correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
was found between the FMA and the Ashworth Scale (r = -0.42). Only a poor correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
was found between the FMA and the modified Barthel Index (r = 0.09).
Note: Correlations were negative because a high score on the FMA indicates normal performance, where as a low score on the Hemispheric StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Scale or the Ashworth Scale indicates normal performance.
Fugl-Meyer and Jaasko (1980) compared the FMA Motor domain to performance on activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living scale in 64 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. more than 6 months after hospital discharge. The activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living scale was comprised of 52 items examining independence in feeding, hygiene, dressing, locomotion, housework, and psychosocial functioning. Excellent correlations were found between the degree of motor impairmentLoss of strength and coordination, decrease in arm or leg movement
as measured by the FMA and the activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living scale (activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living total score r = 0.75; hygiene r = 0.89; locomotion r = 0.76; feeding r = 0.72; and dressing r = 0.76).
Convergent:
Mao et al. (2002) compared the psychometric properties of the Berg Balance Scale, the modified Balance subscore of the FMA, and the Postural Assessment Scale for StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Patients, in 123 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. followed up prospectively 14, 30, 90, and 180 days after strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. onset. Excellent correlations were found using the Spearman correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficient between the scores of the FMA Balance domain and the scores of the Berg Balance Scale at all four time points (ranging from r = 0.86 to r = 0.89), indicating excellent convergent validityA type of validity that is determined by hypothesizing and examining the overlap between two or more tests that presumably measure the same construct. In other words, convergent validity is used to evaluate the degree to which two or more measures that theoretically should be related to each other are, in fact, observed to be related to each other.
.
Rabadi and Rabadi (2006) examined 104 inpatients with acute strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. at a rehabilitation unit. The Action Research Arm Test, the Motor domain upper extremity subscore of the FMA, the NIH StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Scale, the Functional Independence Measure total score, and Functional Independence Measure activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living subscore were administered. Using the Spearman rank correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficient, the Action Research Arm Test and the Motor domain upper extremity subscore of the FMA correlated excellently with one another, both at admission (r = 0.77) and at discharge (r = 0.87). The Motor domain upper extremity subscore of the FMA and the Functional Independence Measure activitiesAs defined by the International Classification of Functioning, Disability and Health, activity is the performance of a task or action by an individual. Activity limitations are difficulties in performance of activities. These are also referred to as function.
of daily living subscore at the time of admission were adequately correlated (r = 0.54).
Arsenault et al. (1988) treated 62 patients with hemiplegiaComplete paralysis of the arm, leg, and trunk on one side of the body that results from damage to the parts of the brain that control muscle movements. Hemiplegia is not a progressive condition, nor is it a disease. with the Bobath approach to treatment for a period of three months. During this time they were evaluated on three occasions. Using Spearman’s Rho, the FMA correlated excellently with the Bobath Assessment of upper extremity pre- (r = 0.73) and post-rehabilitation (r = 0.85), with both measures showing change across time periods.
Dettmann et al. (1987) administered the FMA and the Barthel Index to 15 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Patients were assessed at an average of 2 years post-stroke. Pearson correlations between FMA and Barthel Index scores were excellent for the FMA Total score (r = 0.67), the FMA Motor subscore (r = 0.74), the Motor domain upper extremity subscore (r = 0.75), and the Balance subscore (r = 0.76). In this study, the authors measured walking performance using photographic analysis of gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
pattern and velocity, and postural stability while standing on a force platform. The FMA Motor domain lower extremity subscore correlated well with most of the gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
measurements, and the FMA Balance subscore correlated well with the stability index. The Sensation subscore did not correlate significantly with any of these measures of gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
or upright stability.
Shelton, Volpe, and Reding (2000) compared the FMA to the Functional Independence Measure in 172 inpatients in a strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. rehabilitation hospital within 90 days of strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Total FMA scores were highly correlated with total Functional Independence Measure scores (r = 0.63). The correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
between the FMA Motor domain upper extremity subscore and the Functional Independence Measure self-care scores was excellent (r = 0.61), as was the correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
between the Motor domain lower extremity subscore of the FMA and the Functional Independence Measure mobility score (r = 0.74).
Lin et al. (2004) examined the convergent validityA type of validity that is determined by hypothesizing and examining the overlap between two or more tests that presumably measure the same construct. In other words, convergent validity is used to evaluate the degree to which two or more measures that theoretically should be related to each other are, in fact, observed to be related to each other.
of the FMA Sensation subscore using Spearman’s Rho in 176 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. The FMA Sensation subscore was poor to adequately correlated with the Barthel Index (ranging from r = 0.38 to r = 0.53). FMA Sensation subscore was also poor to adequately correlated to the FMA Motor domain subscore at different post-stroke stages of recovery, indicating low to moderate convergent validityA type of validity that is determined by hypothesizing and examining the overlap between two or more tests that presumably measure the same construct. In other words, convergent validity is used to evaluate the degree to which two or more measures that theoretically should be related to each other are, in fact, observed to be related to each other.
(ranging from r = 0.31 to r = 0.44).
Known groups:
Poole and Whitney (1988) administered the Motor Assessment Scale and the FMA to 30 patients with hemiplegiaComplete paralysis of the arm, leg, and trunk on one side of the body that results from damage to the parts of the brain that control muscle movements. Hemiplegia is not a progressive condition, nor is it a disease.. The FMA lower extremity subscore was able to distinguish between patients who needed assistance in walking better than gaitThe pattern of walking, which is often characterized by elements of progression, efficiency, stability and safety.
speed at speeds less than 0.34 meters/second (r = 0.62).
Bernspang, Asplund, Eriksson, and Fugl-Meyer (1987) administered the FMA to 109 patients within two weeks of having an acute strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. The FMA was found to distinguish between three levels of self-care ability (dependent, partly dependent, and independent).
Responsiveness
Mao et al. (2002) reported a significant change in the modified FMA Balance subscore between times of assessment (14, 30, 90 and 180 days post-stroke). Effect sizes were large in the interval between 14 and 30 days (ES = 0.82) and weakened the further one moved through time from the strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. event (90-180 days was small, ES = 0.33). The overall effect sizeEffect size (ES) is a name given to a family of indices that measure the magnitude of a treatment effect. Unlike significance tests, these indices are independent of sample size. The ES is generally measured in two ways: as the standardized difference between two means, or as the correlation between the independent variable classification and the individual scores on the dependent variable. This correlation is called the "effect size correlation".
(14-180 days) was large (ES = 1.14). When patients were grouped by level of strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. severity, the highest overall FMA Balance subscore effect sizeEffect size (ES) is a name given to a family of indices that measure the magnitude of a treatment effect. Unlike significance tests, these indices are independent of sample size. The ES is generally measured in two ways: as the standardized difference between two means, or as the correlation between the independent variable classification and the individual scores on the dependent variable. This correlation is called the "effect size correlation".
among severe strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. patients was an ES = 1.57.
Van der Lee et al. (2001) examined 22 patients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. who underwent intensive forced-use treatment to improve upper extremity function. A responsivenessThe ability of an instrument to detect clinically important change over time.
ratio (the ratio of the mean change after the experimental intervention and the standard deviation of the mean change during the baseline period) of 2.03 was reported for the Action Research Arm Test and 0.41 for the FMA Motor score. A higher responsivenessThe ability of an instrument to detect clinically important change over time.
ratio indicates greater responsivenessThe ability of an instrument to detect clinically important change over time.
, suggesting that the Action Research Arm Test is more responsive than the FMA to improvement in upper extremity function in response to a forced-use treatment intervention. This was also reflected by the number of patients who improved more than the upper limit of agreement on the Action Research Arm Test during the intervention period, 12 (54.5%), in comparison to only 2 (9.1%) on the FMA, indicating further that the Action Research Arm Test is more responsive to change than the FMA.
Rabadi and Rabadi (2005) assessed the responsivenessThe ability of an instrument to detect clinically important change over time.
of the Action Research Arm Test and the FMA in evaluating recovery of upper extremity function in 104 inpatients with acute strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. The mean change in score from admission to discharge was 10 ± 15 for the Action Research Arm Test and 10 ± 13 for the FMA Motor score. The responsivenessThe ability of an instrument to detect clinically important change over time.
to change as measured by the standard response mean (SRM) was moderate for both the Action Research Arm Test (SRM = 0.68) and for the FMA Motor score (SRM = 0.74).
Lin et al. (2004) examined the responsivenessThe ability of an instrument to detect clinically important change over time.
of the FMA Sensation subscore using the standardized response meanThe standardized response mean (SRM) is calculated by dividing the mean change by the standard deviation of the change scores.
(SRM) in 176 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Small (90-180 days SRM = 0.27; 14-30 days SRM = 0.42; 30-90 days SRM = 0.43) to moderate (14-180 days SRM = 0.67) responsivenessThe ability of an instrument to detect clinically important change over time.
was reported for the FMA Sensation subscore.
Wood-Dauphinee et al. (1990) compared the FMA to the Barthel Index in 167 patients with strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. assessed shortly after admission to the hospital and 5 weeks later. Using Pearson correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
coefficients, the correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
between mean change scores for FMA Upper and lower extremity Motor subscores and total Barthel Index scores was adequate (r = 0.57). Small effect sizes were reported for the FMA Motor scale from admission to 5 weeks post-stroke (0.2 for upper extremity, 0.19 for lower extremity, 0.33 for balance ability, and 0.24 for Total score). The results of this study suggest that the FMA Motor scale has small responsivenessThe ability of an instrument to detect clinically important change over time.
.
Duncan, Lai and Keighley (2000) examined the speed and extent of recovery in 459 patients over the first 6 months following strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Spontaneous improvement was observed for mild, moderate, and severe strokes as measured by the FMA. Within the first month post-stroke, maximum recovery was achieved, and began to plateau around 6 months post-stroke. The recovery curves of the FMA upper extremity and lower extremity Motor subscales’ corresponded to the recover curves of the Barthel Index and the NIH StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Scale. If recovery is defined by achieving an FMA score > 90, then 36.8% of the patients in this study were considered to have recovered.
Shelton et al. (2000) found a moderate correlationThe extent to which two or more variables are associated with one another. A correlation can be positive (as one variable increases, the other also increases - for example height and weight typically represent a positive correlation) or negative (as one variable increases, the other decreases - for example as the cost of gasoline goes higher, the number of miles driven decreases. There are a wide variety of methods for measuring correlation including: intraclass correlation coefficients (ICC), the Pearson product-moment correlation coefficient, and the Spearman rank-order correlation.
between the change in Total FMA score with the change in Total Functional Independence Measure score (r = 0.44). Linear regression analysis demonstrated that with every 24-point increase in Functional Independence Measure score, a 10-point increase in FMA Motor score is observed, indicating that improvement in motor impairmentLoss of strength and coordination, decrease in arm or leg movement
is associated with significant functional recovery. Change in both upper and lower extremity FMA Motor subscores correlated poorly with the change in self-care and mobility Functional Independence Measure subscores (r = 0.23 and r = 0.18, respectively).
Hsueh et al. (2009) examined the responsivenessThe ability of an instrument to detect clinically important change over time.
of the FMA, the StrokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain. Rehabilitation Assessment of Movement (STREAM) and their shortened versions in 50 clients with chronic strokeAlso called a "brain attack" and happens when brain cells die because of inadequate blood flow. 20% of cases are a hemorrhage in the brain caused by a rupture or leakage from a blood vessel. 80% of cases are also know as a "schemic stroke", or the formation of a blood clot in a vessel supplying blood to the brain.. Participants were assessed at two points in time: at admission and at discharge from a rehabilitation program. Both the STREAM and the FMA shortened versions demonstrated a moderate effect sizeEffect size (ES) is a name given to a family of indices that measure the magnitude of a treatment effect. Unlike significance tests, these indices are independent of sample size. The ES is generally measured in two ways: as the standardized difference between two means, or as the correlation between the independent variable classification and the individual scores on the dependent variable. This correlation is called the "effect size correlation".
of 0.53 and 0.51, while the STREAM and FMA demonstrated a small effect sizeEffect size (ES) is a name given to a family of indices that measure the magnitude of a treatment effect. Unlike significance tests, these indices are independent of sample size. The ES is generally measured in two ways: as the standardized difference between two means, or as the correlation between the independent variable classification and the individual scores on the dependent variable. This correlation is called the "effect size correlation".
of 0.45 and 0.38, respectively.
References
-
Arsenault, A. B., Dutil, E., Lambert, J., Corriveau, H., Guarna, F., Drouin, G. (1988). An evaluation of the hemiplegic subject based on the Bobath approach. Part III. A validation study. Scand J Rehabil Med, 20(1), 13-16.
-
Beckerman, Vogelaar, T. W., Lankhorst, G. J., Verbeek, A. L. (1996). A criterion for stability of the function of the lower extremity in stroke patients using the Fugl-Meyer Assessment Scale. Scand J Rehabil Med, 28, 3-7.
-
Berglund, K., Fugl-Meyer, A.R. (1986). Upper extremity function in hemiplegia: A cross validation study of two assessment methods. Scandinavian Journal of Rehabilitation Medicine, 18, 155-157.
-
Bernspang, B., Asplund, K., Eriksson, S., Fugl-Meyer, A. R. (1987). Motor and perceptual impairments in acute stroke patients: effects on self-care ability. Stroke, 18, 1081-1086.
-
Chae, J., Johnston, M., Kim, H., Zorowitz, R. (1995). Admission motor impairment as a predictor of physical disability after stroke rehabilitation. Am J Phys Med Rehabil, 74(3), 218-223.
-
Chae, J., Bethoux, F., Bohine, T., Dobos, L., Davis, T., Friedl, A. (1998). Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke, 29(5), 975-979.
-
Chae, J., Labatia, I., Yang, G. (2003). Upper limb motor function in hemiparesis: Concurrent validity of the arm motor ability test. Am J Phys Med Rehabil, 82, 1-8.
-
Crow, J.L., Harmeling-van der Wel, B.C. (2008). Hierarchical properties of the motor function sections of the Fugl-Meyer Assessment Scale for people after stroke: a retrospective study. Physical Therapy, 88(12), 1554-1567.
-
Dettmann, M. A., Linder, M. T., Sepic, S. B. (1987). Relationships among walking performance, postural stability, and functional assessments of the hemiplegic patient. Amer J Phys Med, 66, 77-90.
-
De Weerdt, W., Harrison, M. A. (1985). Measuring recovery of arm-hand function in stroke patients: a comparison of the Brunnstrom-Fugl-Meyer test and the Action Research Arm test. Physiother Canada, 37, 65-70.
-
Di Fabio, R. P., Badke, M. B. (1990). Relationship of sensory organization to balance function in patients with hemiplegia. Phys Ther, 70(9), 542-548.
-
Duncan, P. W., Lai, S. M. Keighley, J. (2000). Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology, 39(5), 835-41.
-
Duncan, P. W., Propst, M., Nelson, S. G. (1983). Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther, 63(10), 1606-1610.
-
Duncan, P., Richards, L., Wallace, D., Stoker-Yates, J., Pohl, P., and Luchies, C. (1998). A randomized, controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke, 29, 2055-2060.
-
Duncan, P. W., Goldstein, L. B., Horner, R. D., Landsman P. B, Samsa, G. P., Matchar, D. B. (1994). Similar motor recovery of upper and lower extremities after stroke. Stroke, 25, 1181-1188.
-
Ferraro, M., Demaio, J. H., Krol, J., Trudell, C., Rannekleiv, K., Edelstein, L., Christos, P., Aisen, M., England, J., Fasoli, S., Krebs, H., Hogan, N., Volpe, B. T. (2002). Assessing the Motor Status Score: A Scale for the Evaluation of Upper Limb Motor Outcomes in Patients after Stroke. Neurorehabilitation and Neural Repair, 16(3), 283-289.
-
Feyes, H. M., deWeerdt, W. J., Seltz, B. E., Steck G. A. C., Spichinger, R., Vereeck, L. E. (1998). Effect of therapeutic intervention for hemiplegic upper limb in acute phase after stroke. Stroke, 29, 785-792.
-
Feys, H., Van Hees, J., Bruyninck, F., Mercelis, R., De Weerdt, W. (2000). Value of somatosensory and motor evoked potentials in predicting arm recovery after a stroke. J Neurol Neurosurg Psychiatry,68, 323-331.
-
Finch, E., Brooks, D., Stratford, P. W., Mayo, N. E. (2002). Physical Rehabilitations Outcome Measures. A Guide to Enhanced Clinical Decision-Making (2nd ed.). Canadian Physiotherapy Association, Toronto.
-
Fugl-Meyer, A. R., Jaasko, L., Leyman, I., Olsson, S., Steglind, S. (1975). The post-stroke hemiplegic patient: I. A method for evaluation of physical performance. Scandinavian Journal of Rehabilitation Medicine, 975(7), 13-31.
-
Fugl-Meyer, A. R. (1980) Post-stroke hemiplegia assessment of physical properties. Scandinavian Journal of Rehabilitation Medicine, 7, 85-93.
-
Fugl-Meyer, A. R., Jaasko, L. (1980). Post-stroke hemiplegia and ADL-performance. Scand J Rehabil Med Suppl. 7, 140-152.
-
Fulk, G. D., Reynolds, C., Mondal, S., & Deutsch, J. E. (2010). Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil, 91, 1582-1586.
-
Gladstone, D. J., Danells, C. J., Black, S. E. (2002). The Fugl-Meyer Assessment of Motor Recovery after Stroke: A critical review of its measurement properties. Neurorehabilitation and Neural Repair, 16, 232-240.
-
Gowland, C., Van Hullenaar, S., Torresin, W. (1995). Chedoke-McMaster stroke assessment: development, validation and administration manual. Hamilton (ON), Canada: Chedoke-McMaster Hospitals and McMaster University.
-
Gowland, C., Stratford, P., Ward, M. (1993). Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke, 24, 58-63.
-
Hsieh, Y.W., Hsueh, I.P., Chou, Y.T., Sheu, C.F., Hseih, C.L. & Kwakkel, G. (2007). Development and validation of a short form of the Fugl-Meyer motor scale in patients with stroke. Stroke, 38, 3052-3054.
-
Hsueh, I. P., Hsu, M. J., Sheu, C. F., Lee, S., Hsieh, C. L., Lin, J. H. (2009). Psychometric comparisons of 2 versions of the Fugl-Meyer Motor Scale and 2 versions of the Stroke Rehabilitation Assessment of Movement. Neurorehabil Neural Repair, 22, 737.
-
Hsueh, I. P., Mao, H. F., Huang, H. L., Hsieh, C. L. (2001). Comparisons of responsiveness and predictive validity of two balance measures in stroke inpatients receiving rehabilitation [in Chinese]. Formos J Med, 5, 261-268.
-
Kraft, G. H., Fitts, S. S., Hammond, M. C. (1992). Techniques to improve function of the arm and hand. Archives of Physical Medicine and Rehabilitation, 73, 220-227.
-
Kusoffsky, A., Wadell, I., Nilsson, B. Y. (1982). The relationship between sensory impairment and motor recovery in patients with hemiplegia. Scand J Rehabil Med, 14(1), 27-32.
-
Lin, J-H., Hsueh, I-P., Sheu, C-F., Hsieh, C-L. (2004). Psychometric properties of the sensory scale of the Fugl-Meyer Assessment in stroke patients. Clinical Rehabilitation, 18, 391-397.
-
Lin, F. M., Sabbahi, M. (1999). Correlation of spasticity with hyperactive stretch reflexes and motor dysfunction in hemiplegia. Arch Phys Med Rehabil, 80(5), 526-530.
-
Malouin, F., Pichard, L., Bonneau, C., Durand, A., Corriveau, D. (1994) Evaluating motor recovery early after stroke: comparison of the Fugl-Meyer Assessment and the Motor Assessment Scale. Arch Phys Med Rehabil, 75(11), 1206-1212.
-
Mao, H. F., Hsueh, I. P., Tang, P. F., Sheu, C. F., Hsieh, C. L. (2002). Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke, 33, 1022-1027.
-
Nadeau, S., Arsenault, A. B., Gravel, D. Bourbonnais, D. (1999). Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. American Journal of Physical Medicine & Rehabilitation, 78(2), 123-130.
-
Nilsson, L., Carlsson, J., Grimby, G., Nordholm, L. (1998). Assessment of walking balance and sensorimotor performance of hemiparetic patients in the acute stages after stroke. Physiother Theory Pract, 14, 149-157.
-
Platz, T., Pinkowski, C., van Wijck, F., Kim, I-H., di Bella, P., Johnson, G. (2005). Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clinical Rehabilitation, 19, 404-411.
-
Poole, J.L. & Whitney, S.L. (1988). Motor Assessment Scale for stroke patients: Concurrent validity and interrater reliability. Archives of Physical Medicine and Rehabilitation, 69, 195-197.
-
Poole, J. L., Whitney, S. L. (2001). Assessments of Motor Function Post Stroke: A Review. Physical & Occupational Therapy in Geriatrics, 19(2), 1-22.
-
Rabadi, M. H., Rabadi, F. M. (2006). Comparison of the Action Research Arm Test and the Fugl-Meyer Assessment as measures of upper-extremity motor weakness after stroke. Arch Phys Med Rehabil, 87, 962-966.
-
Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. (1993). Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phy Ther, 73, 447-54.
-
Shelton, F. N. A. P., Volpe, B. T., Reding, M. J. (2000). The effect of motor impairment on disability
-
following stroke [abstract]. Stroke, 31(1), 291.
-
Sonde, L., Gip, C., Fernaeus, S.E., Nilsson, C.G., & Viitanen, M. (1998). Stimulation with low frequency (1.7 Hz) transcutaneous electric nerve stimulation (low TENS) increase motor function of the post-stroke paretic arm. Scandinavian Journal of Rehabilitation Medicine, 30, 95-99.
-
Sullivan, K.T., Tilson, J.K., Cen, S.Y., Rose, D.K., Hershberg, J., Correa, A., et al. (2011). Fugl-Meyer Assessment of sensorimotor function after stroke. Standardized training procedure for clinical practice and clinical trials. Stroke, 42, 427-432.
-
Teasell, R., Foley, N. C., & Salter K. (2011). EBRSR: Evidence-Based Review of Stroke Rehabilitation. 13th ed. London (ON): EBRSR.
-
Tyson, S., DeSouza, L. (2002). A systematic review of methods to measure balance and walking post-stroke. Part 1: Ordinal Scales. Physical Therapy Reviews; 7, 173-186.
-
Woodbury, M.L, Velozo, C.A., Richards, L.G., Duncan, P.W., Studenski, S. & Lai, S. (2008). Longitudinal stability of the Fugl-Meyer Assessment of the Upper Extremity. Archives of Physical Medicine and Rehabilitation, 89, 1563-1569.
-
Wood-Dauphinee, S. L., Williams, J. I., Shapiro, S. H. (1990). Examining outcome measures in a clinical study of stroke, Stroke, 21, 731-739.
-
van der Lee, J. H., Beckerman, H., Lankhorst, G. J., Bouter, L. M. (2001). The responsiveness of the Action Research Arm Test and the Fugl-Meyer Assessment Scale in chronic stroke patients. Journal of Rehabilitation Medicine, 33(3), 110-113.
See the measure
How to obtain the FMA?
The FMA can be obtained by following the link below (from the Institute of Rehabilitation Medicine, University of Goteberg, Goteberg, Sweden).
http://www.neurophys.gu.se/sektioner/klinisk_neurovetenskap_och_rehabilitering/neurovetenskap/rehab_med/fugl-meyer/
or through this link form the University of Gothenburg: https://neurophys.gu.se/english/departments/clinical_neuroscience_and_rehabilitation/rehabilitation-medicine/fugl-meyer
A version of the measure is also provided in Fugl-Meyer et al. (1975), and in the book by Dittmar, S. S. and Gresham, G. E. (1997) entitled Functional assessment and outcome measures for the rehabilitation health professional.