Introduction
Constraint-Induced Movement Therapy (CIMT) consists of a set of rehabilitation techniques designed to reduce functional problems in the most affected upper extremity of clients with stroke. This therapy involves constraining movements of the less-affected arm, usually with a sling or mitt for 90% of waking hours, while intensively inducing the use of the more-affected arm. Concentrated, repetitive training of the more-affected limb is usually performed for six hours a day for a two to three week period. Compliance of the patient for the rigorous restraint and training schedule, as well as the required intensity of therapy provided by therapists in a clinical setting, are important issues to consider.
Modified CIMT (mCIMT) is a less intense treatment that involves the same principles as CIMT (i.e. restraint of the less-affected upper extremity and practice of functional activities of the more-affected extremity), but with less intensity than traditional CIMT (i.e. less time). The common therapeutic factor in all CIMT techniques includes concentrated, repetitive tasks with the more-affected arm.
Functional benefits appear to be largely confined to those individuals with some active wrist and hand movement. Studies have explored the efficacy of this intervention for improving functional outcomes post-stroke.
A number of neuro-imaging and transcranial magnetic stimulation studies have shown that CIMT can produce a massive use-dependent cortical reorganization that increases the area of cortex involved in the innervation of movement of the more-affected limb (Taub et al., 1999). In terms of studies examining the effectiveness of this treatment intervention, high quality randomized controlled trials (RCTs) have reported a positive impact for patients with stroke. However, functional benefits appear to be largely confined to those individuals with some active wrist and hand movement.
Patient/Family Information
Authors: Anita Menon, MSc.
What is constraint-induced movement therapy (CIMT)?
After a stroke, regaining strength and function in your weaker arm (the side weakened by the stroke) can be challenging. Constraint-Induced Movement Therapy (CIMT) involves intensive training of the weaker arm while restricting the use of the stronger arm. Specifically, the use of the stronger arm is restricted by the use of a mitten or a sling for much of each day. The idea is to encourage you to use your weaker hand to do daily activities. This therapy has been studied by high quality research studies and has been found beneficial for arm function in some patients- especially those who already have some use of their arm and hand.

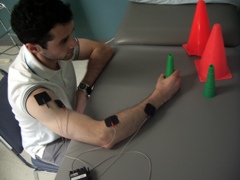
The use of an arm sling during walking training. The sling makes it impossible to use stronger arm. The man must use his weaker arm to hold

Use of a mitten while writing. The mitten makes it impossible to use the good arm. The woman is forced to use her weaker hand to write
Are there different kinds of constraint-induced therapies?
The term “CIMT” is used to describe a newer technique based on older existing techniques that force the patients to use their weaker arm. These older techniques are called “forced-use” therapy. The goal is to intensively train the weaker arm by using it to perform daily tasks such as preparing meals, engage in fun activities such as throwing a ball or fine motor activities such as writing.
More recently, research has looked at the benefit of modified CIMT (mCIMT), which is based on the same principles as CIMT but with less time wearing the restraint and fewer hours of exercise each day.
What is it used for in people with stroke?
The goal of CIMT is to help regain strength and function on the weaker side of the body, typically the side affected by the stroke. CIMT is used for a variety of purposes. Here we describe its use for arm and hand therapy.
Does it work for stroke?
As already mentioned, this therapy has been examined using high quality research studies and has been found beneficial for arm function in some patients after a stroke – especially those who already have some use of their arm and hand.
Although researchers are not exactly sure of how it works, some experts suggest that CIMT affects the brain by enlarging the brain area controlling the weaker arm. Research studies have reported that patients who receive CIMT have better control of their weaker arm and better ability to perform daily activities such as cooking and dressing when compared to people with stroke who received other forms of arm and hand therapy.
What can I expect?
Your therapist will decide with you what regime is most suitable for you. However, CIMT typically requires you to wear either a large mitten or an arm sling on your stronger arm, many hours a day, seven days a week, for about two weeks or more. The mitten or the sling is worn to encourage you to use your weaker arm and hand to do everyday tasks. In addition, the occupational therapist or physical therapist providing the treatment will do exercises with you and may also give you exercises to do on your own or with a family member or friend. While results can vary from person to person, there is scientific evidence that many people who receive this therapy can have improved use of their weaker arm.
Are there any side effects or risks?
CIMT is usually done by a physical therapist or an occupational therapist at a rehabilitation centre or out-patient clinic. However, many of the exercises must be done outside of treatment time. Family members and friends can be very important in helping you do these exercises. Ask your therapist to give you and your friends/family specific information on exercises.
How long is the treatment period?
Intense, repetitive training of the weaker arm is usually given for 90 percent of waking hours (about 13 hours/day) for a 2-week period. This can be done in the clinic, at home, and wherever else it is safe to do so.
An alternative form of CIMT – modified CIMT – is done for fewer hours and possibly, for more weeks. Consult with your therapist or physician who will help you decide which is right for you – CIMT or mCIMT.
This treatment program requires a good deal of self-discipline and commitment. Individuals with stroke tell us it is hard work! Improvement has been shown to be best for those who spend lots of time using the mitten or the sling.
Who provides the treatment?
CIMT is usually provided by a physical therapist or an occupational therapist at a rehabilitation centre or out-patient clinic. However, many of the exercises must be done outside of treatment time. Family members and friends can be very important in helping you do these exercises. Ask your therapist to give you and your friends/family specific information on exercises.
Is constraint-induced movement therapy for me?
CIMT can be of benefit to those who have lost some of the use of their upper limb following stroke. Studies have looked at the benefit for individuals who have had a stroke very recently, over the past couple of months, and those who have experienced a stroke six or more months ago. There is some positive research that suggests that CIMT may be beneficial for certain patients at all of these times.
Clinician Information
Note: When reviewing the findings, it is important to note that they are always made according to randomized clinical trial (RCT) criteria – specifically as compared to a control group. To clarify, if a treatment is “effective” it implies that it is more effective than the control treatment to which it was compared. Non-randomized studies are no longer included when there is sufficient research to indicate strong evidence (level 1a) for an outcome.
The effectiveness of Constraint-Induced Movement Therapy (CIMT) has been explored as an approach to reducing motor impairment and improving motor activity during functional tasks post-stroke. Fifty-one RCTs, 35 of high quality, 12 of fair quality and four of poor quality have examined the effectiveness of CIMT (restraint for 90% of waking hours and 6 hours of upper limb therapy per day for 2 weeks) or modified CIMT (mCIMT – whereby restraint and/or therapy is provided at a lesser intensity than CIMT) in comparison to traditional upper extremity (UE) therapy OR other forms of UE treatment. Studies have explored the use of CIMT or mCIMT at varying phases of stroke recovery: seven RCTs in the acute phase, 16 RCTs in the subacute phase, and 16 RCTs in the chronic phase of stroke recovery. An additional 12 RCTs examined the use of CIMT or mCIMT with populations of patients where the time since stroke was not specific to one period of recovery.
There is evidence to support CIMT and mCIMT as an effective therapy for patients with upper extremity deficits following a stroke. A systematic review and meta-analysis by Corbetta et al. (2010) updated an earlier Cochrane review by Sirtori et al. (2009) of the efficacy of CIMT, mCIMT and forced use techniques for upper extremity rehabilitation in patients with hemiparesis following stroke. A more recent systematic review and meta-analysis by Shi et al. (2011) compared only mCIMT with conventional rehabilitation (e.g. physiotherapy, occupational therapy, neurodevelopmental therapy, neuromuscular facilitation and daily living retraining).
The results from all reviews indicate a significant effect of CIMT, mCIMT or forced use therapy on arm motor function and impairment. The effect on disability is less conclusive, with Sirtori et al. (2009) reporting a moderate effect of CIMT, mCIMT or forced use therapy immediately post-intervention (but not 3 to 6 months post-intervention), Corbetta et al. (2010) reporting no significant effect of CIMT, mCIMT or forced use therapy on disability, and Shi et al. (2011) reporting a significant effect of mCIMT when disability is measured using the Functional Independence Measure, but not the Barthel Index. While all but two studies included in these three reviews are also reviewed on this website, differentiation of the studies according to the particular type of intervention (CIMT or mCIMT) and stage of stroke, as well as the inclusion of other non-randomized studies, has contributed to variation on StrokEngine in reported outcomes at different stroke stages.
The Cochrane review by Sirtori et al. (2009) concluded that restriction of the hand only, and a treatment protocol of no more than 30 hours indicate a significant effect size. Other studies suggest that functional benefits appear to be confined to a subset of stroke patients with existing active wrist and arm movements.
We have reviewed all of the CIMT and mCIMT studies to identify outcomes according to stage of post-stroke recovery (acute, subacute, chronic). Patients less than one month post-stroke were identified as acute, those between 1 and 6 months post-stroke as subacute, and those greater than 6 months post-stroke as chronic. Intensity and duration of treatment were also important factors in influencing outcome, and these details are included in the review of each study.
Results Table
View results table
Outcomes
Acute phase: mCIMT vs. control or alternative interventions
Depression
Not effective
1b
One high quality RCT (Dromerick et al., 2009) examined the effect of mCIMT on depression among patients with acute stroke. This high quality RCT randomized patients with acute stroke to receive ‘standard’ mCIMT (shaping therapy for 2 hours/day and restraint 6 hours/day), ‘intensive’ mCIMT (shaping therapy for 3 hours/day and restraint 90% of waking hours) or conventional UE therapy. There were no significant between-group differences in depression (Geriatric Depression-15 Scale) at post-treatment (14 days) or follow-up (90 days).
Note: The terms ‘standard’ and ‘intensive’ mCIMT were defined by the authors of this study.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is not more effective than conventional rehabilitation for improving depression among patients with acute stroke.
Note: The high quality RCT also found no difference in depression between different intensities of mCIMT.
Two high quality RCTs (Boake et al., 2007; Thrane et al., 2015) have investigated the effect of mCIMT on dexterity in patients with acute stroke.
The first high quality RCT (Boake et al., 2007) randomized patients with acute stroke to receive mCIMT or to receive intensive traditional upper extremity therapy (control group). The mCIMT group received 3 hours of therapy a day and wore a constraint 90% of waking hours. Dexterity was measured with the Grooved Pegboard Test at baseline, at 14-15 days (post-treatment) and at 3 to 4 months post-stroke (follow-up). No significant between-group difference in dexterity was found at either time point.
The second high quality RCT (Thrane et al., 2015) randomized patients with acute stroke to receive mCIMT or usual care. The mCIMT group received rehabilitation for 3 hours/day over 10 consecutive weekdays and wore a mitt on the nonaffected hand for 90% of waking hours. Dexterity was measured with the Nine Hole Peg Test at baseline, 2 weeks (post-treatment) and six months (follow-up). A significant between-group difference in dexterity was found at post-treatment in favour of the mCIMT group compared to the control group. This difference did not remain significant at 6-month follow-up.
Conclusion: There is conflicting evidence (level 4) from 2 high quality RCTs regarding the effect of mCIMT on dexterity. While one high quality RCT found that mCIMT is not more effective than intensive traditional therapy, a second high quality RCT found that mCIMT was more effective than usual care in improving dexterity among patients with acute stroke.
TNote: The significant difference found post-treatment did not remain significant at 6-month follow up.
Functional independence and activities of daily living
Not effective
1a
Three high quality RCTs (Dromerick et al., 2000; Dromerick et al., 2009; Liu et al., 2016) examined the effects of mCIMT on functional independence in patients with acute stroke.
The first high quality RCT (Dromerick et al., 2000) randomized patients with acute stroke to receive mCIMT plus conventional UE therapy or conventional UE therapy alone. The mCIMT group wore a padded mitt 6 hours a day for 14 days and received traditional therapy 2 hours a day. Functional independence in ADL was measured with the Barthel Index and 5 subscales of the Functional Independence Measure (FIM) at discharge from inpatient rehabilitation. A significant between-group difference in upper extremity dressing (FIM), was found in favour of mCIMT compared to conventional UE. There were no other significant between-group differences found in functional independence in ADL (Barthel Index; FIM eating, bathing, grooming and lower body dressing subscales) on discharge.
The second high quality RCT (Dromerick et al., 2009) randomized patients with acute stroke to receive ‘standard’ mCIMT (shaping therapy for 2 hours/day and restraint 6 hours/day), ‘intensive’ mCIMT (shaping therapy for 3 hours/day and restraint 90% of waking hours) or conventional UE therapy. Functional independence was measured with the FIM upper extremity score (sum of the 5 FIM items requiring significant hand and arm use) at baseline, at 14-15 days (post-treatment) and at 3 to 4 months post-stroke (follow-up). No significant between-group differences were found in functional independence at either time point.
Note: The terms ‘standard’ and ‘intensive’ CIMT were defined by the authors of this study.
The third high quality RCT (Liu et al., 2016) randomized patients with acute stroke to receive self-regulated mCIMT (SR-mCIMT), mCIMT or conventional rehabilitation. Participants in the mCIMT groups wore a restraint for 4 hours/day and all participants received individual training for 1 hour/day for 10 days. Functional ability was measured at baseline, 2 weeks (post-treatment) and 4 weeks post-treatment (follow-up) using the Lawton Instrumental Activities of Daily Living Scale (Lawton IADL). There was no signficiant difference in functional ability beween mCIMT and conventional rehabilitation at post-treatment or at follow-up.
Note: There were significant between-group differences in functional ability at post-treatment in favour of SR-mCIMT compared to conventional rehabilitation and in favour of SR-mCIMT compared to mCIMT, but differences did not remain significant at follow-up.
Conclusion: There is strong evidence (level 1a) from 3 high quality RCTs that mCIMT is not more effective than conventional rehabilitation for improving functional independence in patients with acute stroke.
Note: One of the high quality RCTs found a significant difference in the FIM upper extremity dressing score only, in favour of mCIMT compared to conventional rehabilitation.
Note: One high quality RCT found no difference in functional independence between different intensities of mCIMT.
Note: One high quality RCT found that self-regulated mCIMT was more effective than both mCIMT and conventional rehabilitation, although differences did not remain significant at 4 weeks post-treatment.
Motor activity (Upper extremity)
Conflicting
4
Three high quality RCTs (Boake et al., 2007; Thrane et al., 2015; Liu et al., 2016) and 1 fair quality RCT (Page et al., 2005) examined the effects of mCIMT on UE motor activity in patients with acute stroke.
The first high quality RCT (Boake et al., 2007) randomized patients with acute stroke to receive mCIMT or conventional UE therapy. The mCIMT group received therapy 3 hours/day and wore a constraint 90% of waking hours. Upper extremity motor activity was measured with the Motor Activity Log – Amount of Use (MAL-AOU) and – Quality of Movement (MAL-QOM) subscales at baseline, at 14-15 days (post-treatment) and at 3 to 4 months post-stroke (follow-up). There were no significant between-group differences in motor activity at post-treatment or follow up.
The second high quality RCT (Thrane et al., 2015) randomized patients with acute stroke to receive mCIMT or usual care. Participants in the mCIMT group received rehabilitation for 3 hours/day over 10 consecutive weekdays and wore a mitt on the non-affected hand for 90% of waking hours. Upper extremity motor activity was measured according to arm use during functional tasks (arm use ratio) at baseline, 2 weeks (post-treatment) and six months (follow-up). No significant between-group difference were found in functional arm use at either time post.
The third high quality RCT (Liu et al., 2016) randomized patients with acute stroke to receive self-regulated mCIMT (SR-mCIMT), mCIMT or conventional rehabilitation. Participants in the mCIMT groups wore a restraint for 4 hours/day and all participants received individual training for 1 hour/day for 10 days. Upper extremity motor activity was measured at baseline, 2 weeks (post-treatment) and 4 weeks post-treatment (follow-up), using the MAL-AOU and MAL-QOM. At post-treatment there were significant differences in MAL-AOU and MAL-QOM scores in favour of mCIMT compared to conventional rehabilitation; differences did not remain significant at follow-up.
Note: Comparison of SR-mCIMT with conventional rehabilitation showed significant differences in MAL-AOU and MAL-QOM scores at post-treatment and follow-up. Comparison of SR-mCIMT with mCIMT showed significant differences in MAL-AOU scores in favour of SR-mCIMT at post-treatment, but differences did not remain significant at follow-up.
The fair quality RCT (Page et al., 2005) randomized patients with acute stroke to receive mCIMT or conventional rehabilitation. The mCIMT group wore a restraint for 5 hours/day and received 30 minutes of individual therapy 3 days/week for 10 weeks. Motor activity was measured using the Motor Activity Log (MAL) at baseline and 10 weeks (post-treatmeant). At post-treatment the mCIMT group demonstrated a significant improvement in motor activity (MAL).
Note: As this study did not report significant between-group differences these results are not used to determine level of evidence below.
Conclusion: There is conflicting evidence (level 4) among 3 high quality RCTs. While 2 high quality RCTs reported that mCIMT (training 3 hours/day and restraint 90% of waking hours for 2 weeks) is not more effective than conventional rehabilitation, 1 high quality RCT found that mCIMT of less intensity (training 1 hour/day and restraint for 4-5 hours/day for 2 weeks) to be more effective than conventional rehabilitation.
Note: One fair quality RCT also reported significant improvement in motor activity following mCIMT, but did not report between-group differences.
Note: One high quality RCT also found that self-regulated mCIMT was more effective than both mCIMT and conventional rehabilitation, although differences did not remain significant at 1-month follow-up.
Motor function (Upper extremity)
Conflicting
4
Six high quality RCTs (Dromerick et al., 2000; Boake et al., 2007; Dromerick et al., 2009; Thrane et al., 2015; El-Helow et al., 2015; Liu et al., 2016) and 1 fair quality RCT (Page et al., 2005) examined the effectiveness of mCIMT on upper extremity motor function in patients with acute stroke.
The first high quality RCT (Dromerick et al., 2000) randomized patients with acute stroke to receive mCIMT and conventional UE therapy or conventional upper extremity therapy alone. The mCIMT group wore a padded mitt 6 hours/day and received traditional therapy 2 hours/day. Motor function was measured with the Action Research Arm Test (ARAT) at baseline and at post-treatment (14 days). A significant between-group differences in motor function ARAT total score and pinch subscale was found at post-treatment in favour of mCIMT compared to conventional UE therapy alone. No significant between-group differences were found in other ARAT subscales (grasp, grip, gross movement).
The second high quality RCT (Boake et al., 2007) randomized patients with acute stroke to receive mCIMT or conventional UE therapy. The mCIMT group received 3 hours of therapy/day and wore a restraint 90% of waking hours. Motor function was measured with the Fugl Meyer Assessment of Motor Recovery (FMA) and with movements of the affected hand evoked by transcranial magnetic stimulation (TMS) at baseline, at 14-15 days (post-treatment) and at 3 to 4 months post-stroke (follow-up). There was no significant between-group difference in upper extremity motor function at post-treatment or at follow-up.
The third high quality RCT (Dromerick et al., 2009) randomized patients with acute stroke to receive ‘standard’ mCIMT (shaping therapy for 2 hours/day and restraint 6 hours/day), ‘intensive’ mCIMT (shaping therapy for 3 hours/day and restraint 90% of waking hours) or conventional UE therapy. Motor function was measured with the ARAT at baseline, 14 days (post-treatment) and 90 days (follow-up). There were no significant between-group differences in motor function between standard mCIMT and conventional UE therapy at post-treatment or at follow-up. There were significant between-group differences in motor function at post-treatment and follow-up, in favour of standard mCIMT and conventional UE therapy compared to intensive mCIMT.
Note: The terms ‘standard’ and ‘intensive’ CIMT were defined by the authors of this study.
The fourth high quality RCT (Thrane et al., 2015) randomly assigned patients with acute stroke to receive mCIMT or usual care. Participants in the mCIMT group received rehabilitation for 3 hours/day over 10 consecutive weekdays and wore a mitt on the non-affected hand for 90% of waking hours. Motor function was measured with the Wolf Motor Function Test (WMFT) and the FMA-Upper Extremity (FMA-UE) at baseline, 2 weeks (post-treatment) and six months (follow-up). A significant between-group difference was found post-treatment in one subscale of WMFT (performance time) in favour of the mCIMT group compared to the control group. This difference did not remain significant at 6-month follow-up. No significant differences were found in other subscales of the WMFT (functional ability, arm strength and grip strength) or for the FMA-UE at either time point.
The fifth high quality RCT (El-Helow et al., 2015) randomized patients with acute stroke to receive mCIMT or conventional rehabilitation. The mCIMT group wore a restraint for up to 6 hours/day and received shaping intervention for 2 hours/day. Upper extremity motor function was measured with the FMA and ARAT at baseline and 2 weeks (post-treatment). There were significant between-group differences in FMA and ARAT scores at post-treatment, in favour of mCIMT compared to the control group.
The sixth high quality RCT (Liu et al., 2016) randomized patients with acute stroke to receive self-regulated mCIMT (SR-mCIMT), mCIMT or conventional rehabilitation. Participants in the mCIMT groups wore a restraint for 4 hours/day and all participants received individual training for 1 hour/day for 10 days. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and 4 weeks post-treatment (follow-up) using the ARAT (total score, grasp, grip, pinch and gross movement subscales) and the FMA-UE (total score, upper arm, wrist, hand and coordination subscales). At post-treatment there were significant between-group differences in upper extremity motor function (ARAT total, grip, pinch; FMA-UE total score only) in favour of mCIMT compared with conventional rehabilitation; at follow-up there was a significant between-group difference on only one measure of upper extremity motor function (FMA-UE hand), in favour of mCIMT.
Note: Comparison of SR-mCIMT with conventional rehabilitation showed significant between-group differences at post-treatment (ARAT total, pinch; FMA-UE total, upper arm, wrist, hand, coordination) and follow-up (ARAT grip, gross movement; FMA-UE wrist, coordination), in favour of SR-mCIMT. Comparison of SR-mCIMT with mCIMT showed significant differences in only 2 measures of upper extremity motor function at post-treatment (FMA-UE total, coordination), and 3 measures at follow-up (ARAT pinch; FMA-UE hand, coordination), in favour of SR-mCIMT.
The fair quality RCT (Page et al., 2005) randomized patients with acute stroke to receive mCIMT or conventional rehabilitation. The mCIMT group wore a restraint for 5 hours, 5 days/week and received 30 minutes of individual therapy 3 days/week. Motor function was measured with the FMA and the ARAT at baseline and 10 weeks (post-treatment). At post-treatment the mCIMT group demonstrated greater improvement in motor function compared to conventional rehabilitation.
Note: As this study did not report significant between-group differences, these results are not used to determine level of evidence below.
Conclusion: There is conflicting evidence (level 4) among 6 high quality RCTs regarding the effectiveness of mCIMT compared to other interventions. Four of the six high quality RCTs reported significant differences on some measures of upper extremity motor function at post-treatment, in favour of mCIMT compared to conventional rehabilitation. Meanwhile, five of the six high quality RCTs also reported no significant differences on other measures of upper extremity motor function between mCIMT and comparison interventions at post-treatment or follow-up time points.
Note: One high quality RCT found that a mCIMT program of higher intensity (restraint for 90% of waking hours and therapy for 3 hours/day) is less effective than a mCIMT program at a lower intensity (restraint for 6 hours/day and therapy for 2 hours/day) or conventional rehabilitation.
Note: One high quality RCT found that self-regulated mCIMT was more effective than both mCIMT and conventional rehabilitation.
Note: One fair quality RCT also reported significant improvement in motor function following mCIMT, but did not report between-group differences.
One high quality RCT (Dromerick et al., 2009) examined the effects of mCIMT on perception of pain among patients with acute stroke. This high quality RCT randomized patients with acute stroke to receive ‘standard’ mCIMT (shaping therapy for 2 hours/day and restraint 6 hours/day), ‘intensive’ mCIMT (shaping therapy for 3 hours/day and restraint 90% of waking hours) or conventional UE therapy. Pain was measured with the Wong-Baker Faces Scale at baseline, 14 days (post-treatment) and 90 days (follow-up). No significant between-group differences were found in pain for any group at post-treatment or follow-up.
Note: The terms ‘standard’ and ‘intensive’ CIMT were defined by the authors of this study.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is not more effective than conventional rehabilitation for alleviating pain among patients with acute stroke.
Note: The high quality RCT also found no difference in pain between different intensities of mCIMT.
Stroke outcomes
Not effective
1A
Two high quality RCTs (Dromerick et al., 2009; Thrane et al., 2015) examined the effects of mCIMT on stroke outcomes in patients with acute stroke.
The high quality RCT (Dromerick et al., 2009) randomized patients with acute stroke to receive ‘standard’ mCIMT (shaping therapy for 2 hours/day and restraint 6 hours/day), ‘intensive’ mCIMT (shaping therapy for 3 hours/day and restraint 90% of waking hours) or conventional UE therapy. Stroke outcomes were measured at baseline, post-treatment (14 days) and follow-up (90 days), using the Stroke Impact Scale (SIS) hand function subtest. There were no significant differences in self-perception of hand function (SIS hand function subscale) at post-treatment (14 days) or follow-up (90 days) between standard mCIMT and conventional UE therapy. However, there were significant between-group differences in SIS hand function scores at follow-up (90 days), in favour of both standard CIMT and conventional UE therapy when compared with high-intensity CIMT.
Note: The terms ‘standard’ and ‘intensive’ CIMT were defined by the authors of this study.
The second high quality RCT (Thrane et al., 2015) randomly assigned patients with acute stroke to receive modified CIMT or usual care. Participants in the mCIMT group received rehabilitation for 3 hours/day over 10 consecutive weekdays and wore a mitt on the nonaffected hand for 90% of waking hours. Stroke outcomes were measured with the SIS hand function, ADL/IADL, participation/role function and global perception of recovery subtests at baseline, 2 weeks (post-treatment) and six months (follow-up). There were no significant between-group differences in stroke outcomes at post-treatment (2 weeks) or at 6-month follow-up.
Conclusion: There is strong evidence (level 1a) from 2 high quality RCTs that mCIMT is not more effective than conventional rehabilitation for improving stroke outcomes in patients with acute stroke.
Note: One quality RCT found that high-intensity mCIMT (restraint for 90% of waking hours and therapy for 3 hours/day) is less effective than low-intensity mCIMT (restraint for 6 hours/day and therapy for 2 hours/day) or conventional rehabilitation for improving self-perception of hand function.
Subacute phase: CIMT vs. control or alternative interventions
Finger dexterity
Not effective
2A
A fair quality RCT (Yoon et al., 2014) has examined the effect of CIMT on dexterity among patients with subacute stroke. This fair quality RCT randomized patients with subacute stroke to receive CIMT, CIMT + mirror therapy, or a control group that received occupational therapy and a self-exercise program. Finger dexterity was measured at baseline and 2 weeks (post-treatment) using the Nine Hole Peg Test (NHPT). There was no significant difference in finger dexterity at post-treatment between CIMT and the control group.
Note: At post-treatment the CIMT + mirror therapy group showed significantly better finger dexterity than the CIMT group and the control group.
Conclusion: There is limited evidence (level 2a) from 1 fair quality RCT that CIMT is not more effective than a comparison intervention (occupational therapy) for improving finger dexterity among patients with subacute stroke.
Note: This fair quality RCT found that CIMT with mirror therapy was more effective than CIMT alone for improving finger dexterity.
Functional independence
Effective
2A
One fair quality RCT (Yoon et al., 2014) has examined the effect of CIMT on functional independence among patients with subacute stroke. This fair quality RCT randomized patients with subacute stroke to receive CIMT, CIMT + mirror therapy, or a control group that received occupational therapy and a self-exercise program. Functional independence was measured at baseline and 2 weeks (post-treatment) using the Korean version of the modified Barthel Index (K-mBI). At post-treatment there was a significant between-group difference in functional independence, in favour of CIMT compared to the control group.
Note: At post-treatment the CIMT + mirror therapy group also showed significantly better K-mBI scores than the control group. There were no significant differences between CIMT and CIMT + mirror therapy at post-treatment.
Conclusion: There is limited evidence (level 2a) from 1 fair quality RCT that CIMT is more effective than comparison interventions (occupational therapy) for improving functional independence among patients with subacute stroke.
A fair quality RCT (Yoon et al., 2014) has examined the effect of CIMT on grip strength among patients with subacute stroke. This fair quality RCT randomized patients with subacute stroke to receive CIMT, CIMT + mirror therapy, or a control group that received occupational therapy and a self-exercise program. Grip strength was measured at baseline and 2 weeks (post-treatment). There was a significant between-group difference in grip strength at post-treatment, in favour of CIMT compared to the control group.
Note: At post-treatment the CIMT + mirror therapy group showed significantly better grip strength than the CIMT group and the control group.
Conclusion: There is limited evidence (level 2a) from 1 fair quality RCT that CIMT is more effective than a comparison intervention (occupational therapy) for improving grip strength among patients with subacute stroke.
Note: The fair quality RCT found that CIMT with mirror therapy was more effective than CIMT alone for improving grip strength.
Manual dexterity
Effective
2A
A fair quality RCT (Yoon et al., 2014) examined the effect of CIMT on dexterity among patients with subacute stroke. This fair quality RCT randomized patients with subacute stroke to receive CIMT, CIMT + mirror therapy, or a control group that received occupational therapy and a self-exercise program. Manual dexterity was measured at baseline and 2 weeks (post-treatment) using the Box and Block Test (BBT). There was a significant between-group difference in manual dexterity at post-treatment, in favour of CIMT compared to the control group.
Note: At post-treatment the CIMT + mirror therapy group showed significantly better manual dexterity than the CIMT group and the control group.
Conclusion: There is limited evidence (level 2a) from 1 fair quality RCT that CIMT is more effective than a comparison intervention (occupational therapy) for improving manual dexterity among patients with subacute stroke.
Note: The fair quality RCT found that CIMT with mirror therapy was more effective than CIMT alone for improving manual dexterity.
Motor function (Upper extremity)
Not effective
1b
One high quality RCT (Sawaki et al., 2008) and 1 fair quality RCT (Yoon et al., 2014) have examined the effect of CIMT on upper extremity (UE) motor function in patients with subacute stroke.
The high quality RCT (Sawaki et al., 2008) randomized patients with subacute stroke to receive CIMT or usual care. The CIMT group wore a padded mitt on the less-affected limb for at least 90% of waking hours and performed intensive therapy for 6 hours/day, 5 days/week for 2 weeks. Upper extremity motor function was measured using the Wolf Motor Function Test (WMFT) at baseline, 2 weeks (post-treatment) and at 4 months (follow-up). There was a significant between-group difference in only one measure of upper extremity motor function (WMFT grip strength), in favour of the CIMT group compared to the control group. There were no significant between-group differences in other measures of UE motor function (WMFT weight and time-based measures).
The fair quality RCT (Yoon et al., 2014) randomized patients with subacute stroke to receive CIMT, CIMT + mirror therapy, or a control group that received occupational therapy and a self-exercise program. Upper extremity motor function was measured at baseline and 2 weeks (post-treatment) using the Wolf Motor Function Test (WMFT) and the Fugl-Meyer Assessment (FMA) – total score and Upper Extremity score (FMA-UE). At post-treatment there was a significant between-group difference in one measure of upper extremity motor function (WMFT), in favour of CIMT compared to the control group.
Note: At post-treatment the CIMT + mirror therapy group also showed significantly better WMFT scores than the control group.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT and 1 fair quality RCT that CIMT is not more effective than comparison interventions (usual care, CIMT with mirror therapy, occupational therapy) in improving UE motor function in patients with subacute stroke.
Note: However the high quality RCT found a difference in WMFT grip strength in favour of CIMT vs. usual care; the fair quality RCT found differences in WMFT scores in favour of CIMT vs. OT and in favour of CIMT + mirror therapy vs. OT.
Stroke recovery
Not effective
2A
One fair quality RCT (Yoon et al., 2014) has examined the effect of CIMT on stroke recovery among patients with subacute stroke. This fair quality RCT randomized patients with subacute stroke to receive CIMT, CIMT + mirror therapy, or a control group that received occupational therapy and a self-exercise program. Stroke recovery was measured at baseline and 2 weeks (post-treatment) using the Brunnstrom stages of stroke recovery. There were no significant between-group differences in stroke recovery at post-treatment.
Conclusion: There is limited evidence (level 2a) from 1 fair quality RCT that CIMT is not more effective than comparison interventions (CIMT and mirror therapy, occupational therapy) for improving stroke recovery among patients with subacute stroke.
Subacute phase: mCIMT vs. control or alternative interventions
Four high quality RCTs (Myint et al., 2008; Hammer & Lindmark, 2009b; Brunner, Skouen & Strand, 2012; Treger et al., 2012) have examined the effect of mCIMT on finger dexterity in patients with subacute stroke.
The first high quality RCT (Myint et al., 2008) randomized patients with subacute stroke to receive either mCIMT or conventional occupational and physical therapy. The mCIMT group wore a shoulder sling on the less-affected extremity for 90% of waking hours and received 4 hours daily therapy. Finger dexterity was measured using the Nine Hole Peg Test (NHPT) at baseline, 10 days (post-treatment) and follow-up (12 weeks). There were significant between-group differences in dexterity at post-treatment and follow-up, in favour of mCIMT compared to conventional rehabilitation.
The second high quality RCT (Hammer & Lindmark, 2009b) randomized patients with subacute stroke to a ‘forced use’ group or a control group that received conventional rehabilitation. The forced use group wore a sling to promote forced use of the paretic upper limb for up to 6 hours/day, 5 days/week. Finger dexterity was measured using the 16 Hole Peg Test at baseline, 2 weeks (post-treatment), 1 month (follow-up A) and 3 months (follow-up B). There were no significant between-group differences in dexterity at any time point.
The third high quality RCT (Brunner, Skouen & Strand, 2012) randomized patients with subacute stroke to receive mCIMT or bimanual task-related training. The mCIMT group wore a restraint for 4 hours/day in addition to therapist-directed training for 4 hours/week and self-directed training for 2-3 hours/day for 4 weeks. Finger dexterity was measured at baseline, 4 weeks (post-treatment) and 3-month follow-up using the Nine Hole Peg Test (NHPT). There were no significant between-group differences in finger dexterity at any time point.
The fourth high quality RCT (Treger et al., 2012) randomly assigned patients with subacute stroke to receive mCIMT or conventional rehabilitation. The mCIMT group restrained the non-affected hand during 1-hour rehabilitation sessions and wore a mitten for up to 4 hours each weekday for 2 weeks. Dexterity was measured by a peg task and a ball grasp, carry and release task modified from the Manual Function Test at baseline and 4 weeks. There were significant between-group differences in dexterity at 4 weeks, in favour of mCIMT compared to conventional rehabilitation.
Conclusion: There is conflicting evidence (level 4) between 2 high quality RCTs that found mCIMT to be more effective than comparison interventions (conventional rehabilitation), and a third high quality RCT that found mCIMT was not more effective than the comparison interventions (bimanual task training) for improving finger dexterity in patients with subacute stroke. A fourth high quality RCT found that forced use therapy with conventional rehabilitation was not more effective than conventional rehabilitation alone for improving finger/manual dexterity among patients with subacute stroke.
Functional independence and activities of daily living
Conflicting
4
Three high quality RCTs (Myint et al., 2008, Azab et al., 2009; Treger et al., 2012) examined the effects of mCIMT on functional independence and activities of daily living (ADLs) in patients with subacute stroke.
The first high quality RCT (Myint et al., 2008) randomized patients with subacute stroke to receive mCIMT or conventional occupational and physical therapy. The mCIMT group received 4 hours daily of therapy and wore a shoulder sling on the less-affected extremity for 90% of waking hours. Functional independence was measured using the modified Barthel Index (mBI) at baseline, 2 weeks (post-treatment) and 12 weeks (follow-up). There were no significant between-group differences in ADLs at either time point.
The second high quality RCT (Azab et al., 2009) randomized patients with subacute stroke to receive mCIMT or standard rehabilitation. The mCIMT group wore a mitt on the unaffected hand for 6-7 hours per day and both groups received physical therapy and occupational therapy for 40 mins/session, 3 times/week for the treatment period. Functional independence was measured using the Barthel Index (BI) at baseline, 4 weeks (post-treatment) and 6 months (follow-up). There was a significant between-group difference in functional independence at both time points, in favour of mCIMT compared to standard rehabilitation.
The third high quality RCT (Treger et al., 2012) randomly assigned patients with subacute stroke to receive mCIMT or conventional rehabilitation. The mCIMT group restrained the nonaffected hand during 1-hour rehabilitation sessions and wore a mitten for up to 4 hours each weekday for 2 weeks. Functional independence was measured according to spoon use over 30 seconds, at baseline and at 4 weeks. There was a significant between-group difference in spoon use at 4 weeks, in favour of mCIMT compared to conventional rehabilitation.
Conclusion: There is conflicting evidence (level 4) regarding the effect of mCIMT on functional independence in patients with subacute stroke. One high quality RCT reported that a short-term (2-week) high-intensity mCIMT program was not more effective than conventional rehabiliation, whereas a second high quality RCT reported that a longer (4-week), low-intensity mCIMT program was more effective than conventional rehabilitation. A third high quality RCT also reported that low-intensity mCIMT was more effective than conventional rehabilitation, using a non-standardised measure of functional rehabilitation (spoon use).
Grip strength
Not effective
1b
One high quality RCT (Hammer & Lindmark, 2009b) investigated the effect of mCIMT on hand strength in patients with subacute stroke. This high quality RCT randomized patients with subacute stroke to a ‘forced use’ group or a control group that received conventional rehabilitation. The forced use group wore a sling to promote forced use of the paretic upper limb for up to 6 hours/day, 5 days/week for 2 weeks. Isometric grip strength was measured at baseline, 2 weeks (post-treatment), 1 month (follow-up A) and 3 months (follow-up B). There was no significant between-group difference in grip strength at any time point.
Conclusion: There is moderate evidence (level 1b) from one high quality RCT that mCIMT is not more effective than comparison interventions (conventional rehabilitation) for improving grip strength among patients with subacute stroke.
Motor activity (Upper extremity)
Not effective
1a
Five high quality RCTs (Page et al., 2002; Myint et al., 2008; Hammer & Lindmark, 2009a; Brogardh et al., 2009b; Brogardh & Lexell, 2010 – follow-up study; Brunner, Skouen & Strand, 2012) and one fair quality RCT (Page et al., 2001) examined the effects of mCIMT on UE motor activity in patients with subacute stroke.
The first high quality RCT (Page et al., 2002) randomized patients with subacute stroke to receive mCIMT, traditional therapy, or no treatment. The mCIMT group wore a restraint for 5 hours/day and received 30 minutes each of physical therapy and occupational therapy 3 times/week. Upper extremity motor activity was measured using the Motor Activity Log (MAL) at baseline and at 10 weeks (post-treatment). There were no significant between-group differences in MAL scores at post-treatment.
The second high quality RCT (Myint et al., 2008) randomized patients with subacute stroke to receive either mCIMT or conventional occupational and physical therapy. The mCIMT group received therapy for 4 hours/day and wore a shoulder sling on the less-affected extremity for 90% of waking hours during the 10-day treatment period. Upper extremity motor activity was measured using the MAL Amount of Use (MAL-AOU) and Quality of Movement (MAL-QOM) at baseline, 2 weeks (post-treatment) and 12 weeks (follow-up). There were significant between-group differences in motor activity at both time points, in favour of mCIMT compared to conventional therapy.
The third high quality RCT (Hammer & Lindmark, 2009a) randomized patients with subacute stroke to a ‘forced use’ group or a control group that received conventional rehabilitation alone. The forced use group wore a sling to promote forced use of the paretic upper limb for up to 6 hours/day, 5 days/week for 2 weeks. Upper extremity motor activity was measured using the MAL-AOU and MAL-QOM at baseline, 2 weeks (post-treatment), 1 month (follow-up A) and 3 months (follow-up B). There were no significant between-group differences in motor activity at any time point.
The fourth high quality RCT (Brogardh et al., 2009b) randomized patients with subacute stroke to a mCIMT group that wore a mitt on the less affected arm for 90% of waking hours, or a group that did not wear a mitt. Both groups received 3 hours of therapy for the affected arm for 12 days. Upper extremity motor activity was measured using the MAL at baseline, 2 weeks (post-treatment) and 3 months (follow-up). There were no significant between-group differences in motor activity at either time point.
Further to the study by Brogardh et al, 2009b (Brogardh & Lexell, 2010), no significant between-group differences in upper extremity motor activity (MAL-AOU, MAL-QOM) were seen at 12 months follow-up.
The fifth high quality RCT (Brunner, Skouen & Strand, 2012) randomized patients with subacute stroke to receive mCIMT or bimanual task-related training. The mCIMT group wore a restraint for 4 hours/day in addition to therapist-directed training for 4 hours/week and self-directed training for 2-3 hours/day for 4 weeks. Upper extremity motor activity was measured at baseline, 4 weeks (post-treatment) and 3-month follow-up using the MAL-AOU and MAL-QOM. There were no significant between-group differences in upper extremity motor activity at any time point.
The fair quality RCT (Page et al., 2001) randomized patients with subacute stroke to receive mCIMT, conventional rehabilitation or no therapy. mCIMT comprised 30 minutes each of physiotherapy and occupational therapy 5 days/week for 10 weeks and restraint of the less affected arm 5 hours/day, 5 days/week for 10 weeks. Upper extremity motor activity was measured using the MAL-AOU and MAL-QOM. The mCIMT group demonstrated improved motor activity at post-treatment, whereas the other two groups did not demonstrate substantial improvement.
Note: Statistical data and between-group differences were not reported; accordingly this study is not included in determining level of evidence in the conclusion below.
Conclusion: There is strong evidence (level 1a) from 4 high quality RCTs that mCIMT or forced-use therapy is not more effective than control therapies (e.g. no treatment, conventional rehabilitation, mCIMT training with no mitt use, bimanual task training) for improving UE motor activity in patients with subacute stroke.
Note: However, one high quality RCT did find significant between-group differences in UE motor activity in favour of mCIMT compared to conventional therapy. Also, one fair quality RCT demonstrated improved motor activity at post-treatment for the mCIMT group but as statistical data and between-group differences were not reported, this study is not included in determining level of evidence.
Motor function (Upper extremity)
Not effective
1a
Five high quality RCTs (Page et al., 2002; Myint et al., 2008; Hammer & Lindmark, 2009b; Brogardh et al., 2009b; Brogardh & Lexell, 2010 – follow-up study; Brunner, Skouen & Strand, 2012) and one fair quality RCT (Page et al., 2001) examined the effects of mCIMT on upper extremity (UE) motor function in patients with subacute stroke.
The first high quality RCT (Page et al., 2002) randomized patients with subacute stroke to receive mCIMT, traditional therapy, or no treatment. The mCIMT group wore a restraint for 5 hours/day and received 30 minutes each of physical therapy and occupational therapy 3 times/week for 10 weeks. Motor function was assessed using the Fugl-Meyer Assessment (FMA) and the Action Research Arm Test (ARAT) at baseline, 10 weeks (post-treatment). At post-treatment (10 weeks) there was a significant between-group difference in FMA scores in favour of mCIMT compared to traditional therapy and no therapy at post-treatment. There were no significant differences in ARAT scores between any groups at post-treatment.
The second high quality RCT (Myint et al., 2008) randomized patients with subacute stroke to receive either mCIMT or conventional occupational and physical therapy. The mCIMT group wore a shoulder sling on the less-affected extremity for 90% of waking hours and received 4 hours daily therapy for 10 days. Upper extremity motor function was measured using the ARAT and Functional Test of the Hemiparetic Upper Extremity (FTHUE) at baseline, 10 days (post-treatment) and 12 weeks (follow-up). There were significant between-group differences in ARAT (grasp, grip, pinch, gross movement) and FTHUE scores at post-treatment, and in ARAT (total, grip) and FTHUE scores at follow-up, in favour of mCIMT compared to conventional therapy.
The third high quality RCT (Hammer & Lindmark, 2009b) randomized patients with subacute stroke to a ‘forced use’ group or a control group that received conventional rehabilitation. The forced use group wore a sling to promote forced use of the paretic upper limb for up to 6 hours/day, 5 days/week for 2 weeks. Upper extremity motor function was measured using the FMA-UE, ARAT and Motor Assessment Scale at baseline, 2 weeks (post-treatment), 1 month (follow-up A) and 3 months (follow-up B). There were no significant between-group differences in upper extremity motor function at any time point.
The fourth high quality RCT (Brogardh et al., 2009b) randomized patients with subacute stroke to a mCIMT group that wore a mitt on the less affected arm for 90% of waking hours, or a group that did not wear a mitt. Both groups received 3 hours of therapy for the affected arm for 12 days. Upper extremity motor function was measured using the modified Motor Assessment Scale and the Sollerman Hand Function Test (SHFT) at baseline, 2 weeks (post-treatment), 3 months and 12 months (follows-up). There were no significant between-group differences in upper extremity motor function at post-treatment or at follow-up (see Brogardh & Lexell, 2010).
The fifth high quality RCT (Brunner, Skouen & Strand, 2012) randomized patients with subacute stroke to receive mCIMT or bimanual task-related training. The mCIMT group wore a restraint for 4 hours/day in addition to therapist-directed training for 4 hours/week and self-directed training for 2-3 hours/day for 4 weeks. Upper extremity motor function was measured at baseline, 4 weeks (post-treatment) and 3-month follow-up using the ARAT. There were no significant between-group differences in upper extremity motor function at any time point
The fair quality RCT (Page et al., 2001) randomized patients with subacute stroke to receive mCIMT, conventional rehabilitation or no therapy. mCIMT comprised 30 minutes each of physiotherapy and occupational therapy 5 days/week for 10 weeks and restraint of the less affected arm 5 hours/day, 5 days/week for 10 weeks. Upper extremity motor function was measured using the ARAT, FMA and Wolf Motor Function Test (WMFT) at baseline and 10 weeks (post-treatment). The mCIMT group demonstrated substantial improvements in motor function whereas the other groups did not demonstrate substantial improvements on any measure of upper extremity motor function. Statistical data and between-group differences were not reported; accordingly this study is not included in determining level of evidence in the conclusion below.
Conclusion: There is strong evidence (level 1a) from 4 high quality RCTs that mCIMT or forced use therapy is not more effective than comparison interventions (conventional rehabilitation, no treatment, mCIMT training with no mitt use or bimanual task training) for improving upper extremity motor function among patients with subacute stroke. However, 1 of these high quality RCTs found results in favour of mCIMT on one measure of upper extremity motor function (FMA) but not another (ARAT); and another highquality RCTfound that mCIMT is more effective than control therapy (e.g. conventional rehabilitation).
Note: Studies varied in constraint intensity (from 4 hours/day to 90% of waking hours), frequency of therapy (from 3 to 20 hours/week) and intervention duration (from 10 days to 10 weeks), which is likely to account for discrepancies in results among studies.
Note: One fair quality RCT demonstrated improved motor function at post-treatment for the mCIMT group but as statistical data and between-group differences were not reported, this study is not included in determining level of evidence.
Spasticity
Not effective
1B
One high quality RCT (Hammer & Lindmark, 2009b) examined the effects of mCIMT on upper extremity spasticity in patients with subacute stroke. This high quality study randomized patients with subacute stroke to a ‘forced use’ group that wore a sling on the less affected arm for up to 6 hours/day, 5 days a week for 2 weeks, or to a control group that received conventional rehabilitation alone. Upper extremity spasticity was measured using the Modified Ashworth Scale at baseline, 2 weeks (post-treatment), 1 month (follow-up A) and 3 months (follow-up B). There were no significant between-group differences in upper extremity spasticity at any time point.
Conclusion: There is moderate evidence (level 1b) from one high quality RCT that mCIMT is not more effective than conventional rehabilitation for improving upper extremity spasticityin patients with subacute stroke.
Chronic phase: CIMT vs. control or alternative interventions
Functional independence
Not effective
1B
One high quality RCT (Huseyinsinoglu, Ozdincler, & Krespi, 2012) investigated the effect of CIMT on functional independence in patients with chronic stroke. This high quality RCT randomized patients with chronic stroke to receive CIMT or Bobath Concept therapy. Functional independence was measured using the Functional Independence Measure (FIM self cares and total score) at baseline and 2 weeks (post-treatment). There were no significant between-group differences in functional independence at post-treatment.
Conclusion: There is moderate (level 1b) evidence from 1 high quality RCT that CIMT is not more effective than a comparison intervention (Bobath Concept therapy) for improving functional independence among patients with chronic stroke.
One high quality RCT (Suputtitada et al., 2004) investigated the effect of CIMT on hand strength in patients with chronic stroke. This high quality RCT randomized patients with chronic stroke to receive CIMT and affected-UE training or bimanual-UE training without restraint. Pinch strength and grip strength were measured by dynamometer at baseline and 2 weeks (post-treatment). At post-treatment there were significant between-group differences in pinch strength only, in favour of the CIMT group compared to the control group.
Conclusion: There is moderate evidence (level 1b) from one high quality RCT that CIMT is more effective than comparison interventions (bimanual upper extremity training) for improving pinch strength – but not grip strength – among patients with chronic stroke.
Kinematics (Upper extremity)
Insufficient evidence
5
One pre-post study (Richards et al., 2008) investigated the effect of CIMT on upper extremity kinematics with 3 patients with chronic stroke and ataxia. This pre-post study assigned patients to wear a mitten restraint during 90% of waking hours. Patients 1 and 2 received therapy 6 hours a day (CIMT) while patient 3 received therapy 3 hours a day (mCIMT). All participants demonstrated improved kinematic reaching values post-intervention, with no significant between-patient differences.
Note: Patients 1 and 2 improved on all kinematic measures: maximum velocity and time to maximum velocity increased, while index of curvature, number of peaks in the velocity profile, and trunk movement decreased. Participant 3 improved on some kinematic measures (smoother velocity profile, increased time to maximum velocity and decreased number of peaks in the velocity profile) but not all (decreased maximum velocity, increased index of curvature).
Conclusion: There is an insufficient evidence (level 5) comparing CIMT and control therapies on upper extremity kinematics in patients with chronic stroke. However, 1 non-experimental study found that CIMT is effective for improving reach kinematics in patients with chronic stroke.
Motor activity (Upper extremity)
Effective
1b
One high quality RCT (Huseyinsinoglu, Ozdincler, & Krespi, 2012), two fair quality RCTs (Wittenberg et al., 2003; Brogårdh & Sjülund, 2006; Brogårdh et al., 2009a – follow-up study) and 2 non-experimental studies (Taub et al., 2006; Kunkel et al., 1999) examined the effectiveness of CIMT on motor activity in patients with chronic stroke.
The high quality RCT (Huseyinsinoglu, Ozdincler, & Krespi, 2012) randomized patients with chronic stroke to receive CIMT or Bobath Concept therapy. Upper extremity motor activity was measured using the Motor Activity Log – Amout of Use (MAL-AOU) and – Quality of Movement (MAL-QOM) at baseline and 2 weeks (post-treatment). There was a significant between-group difference in MAL-AOU and MAL-QOM scores at post-treatment, in favour of CIMT compared to Bobath Concept therapy.
The first fair quality RCT (Wittenberg et al., 2003) randomized patients with chronic stroke to receive either CIMT and task-oriented training or task-oriented training alone. Upper extremity motor activity was measured using the MAL at baseline and 10 days (post-treatment). There were significant gains in MAL scores at post-treatment, in favour of CIMT + task-oriented training compared to task-oriented training alone.
The second fair quality RCT (Brogårdh & Sjülund, 2006) provided patients with chronic stroke with a 2-week CIMT program, then randomized patients to prolonged mitt use for a further 3 months, or no further treatment. All participants demonstrated improved MAL-AOU and MAL-QOM scores after 2 weeks of CIMT, however no significant between-group differences were reported at 3 months.
Note: The authors reported that the small sample size was a limitation of this study.
A 4-year follow-up to this study (Brogårdh et al., 2009a) found a significant improvement in upper extremity motor activity from baseline to 4 years. However, comparison from post-treatment time points (2 weeks and 3 months) to 4-year follow-up revealed a significant decrease in motor activity.
A controlled clinical trial (Taub et al., 2006) assigned patients with chronic stroke to a CIMT group or a control group that received time-matched and interaction-matched physical, cognitive and relaxation exercises. Upper extremity motor activity was measured using the MAL-QOM subtest and the Upper Extremity Actual Amout of Use test (AAUT) at baseline, 2 weeks (post-treatment), 4 weeks (follow-up A) and 2 years (follow-up B). There was a significantly greater improvement in upper extremity motor activity at post-treatment, in favour of CIMT compared to the control group. No significant between-group differences were reported at follow-up time points.
A pre-post study without multiple baselines (Kunkel et al., 1999) assigned patients with chronic stroke to receive CIMT. Upper extremity motor activity was measured using the MAL and AAUT at baseline, 2 weeks (post-treatment) and 3 months (follow-up). Significant improvements in upper extremity motor activity were noted at both time points.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT, 1 fair quality RCT and 1 non-experimental study that CIMT is more effective than comparison interventions (e.g. Bobath Concept therapy, task-oriented training, time-matched physical/cognitive/relaxation exercises) for improving upper extremity motor activity in patients with chronic stroke. Further, 1 fairquality RCTand 1 non-experimental study found significant improvements in motor activity following CIMT.
Note: One fair quality RCT found no benefit to prolonged mitt use following CIMT.
Motor function (Upper extremity)
Conflicting
4
Three high quality RCTs (Suputtitada et al., 2004; Huseyinsinoglu, Ozdincler, & Krespi, 2012; Abo et al., 2014), 2 fair quality RCTs (Wittenberg et al., 2003; Brogårdh & Sjülund, 2006; Brogårdh et al., 2009a – follow-up study) and 2 non-experimental studies (Taub et al., 2006; Kunkel et al., 1999) examined the effects of CIMT on upper extremity motor function in patients with chronic stroke.
The first high quality RCT (Suputtitada et al., 2004) randomized patients with chronic stroke to receive CIMT and affected-UE training or bimanual-UE training without restraint. Upper extremity motor function was measured using the Action Research Arm Test (ARAT). At post-treatment, there were significant between-group differences in upper extremity motor function, in favour of CIMT compared to the control group.
The second high quality RCT (Huseyinsinoglu, Ozdincler, & Krespi, 2012) randomized patients with chronic stroke to receive CIMT or Bobath Concept therapy. Upper extremity motor function was measured at baseline and 2 weeks (post-treatment) using the Wolf Motor Function Test – Functional Ability and – Performance Time (WMFT-FA, WMFT-PT) subtests. There was no significant between-group difference in upper extremity motor function at post-treatment.
The third high quality RCT (Abo et al., 2014) randomized patients with chronic stroke to receive CIMT or low-frequency rTMS with intensive OT (NEURO). Upper extremity motor function was measured at baseline and 15 days (post-treatment) using the FMA and WMFT-FA and WMFT-PT. There were significant between-group differences in FMA and WMFT-FA scores at post-treatment, in favour of NEURO compared to CIMT.
The first fair quality RCT (Wittenberg et al., 2003) randomized patients with chronic stroke to receive CIMT and task-oriented training or task-oriented training alone. Upper extremity motor function was measured at baseline and at 2 weeks (post-treatment) using the WMFT and the Assessment of Motor and Process Skills (AMPS), and using Transcranial Magnetic Stimulation and Positron Emission Tomography (PET) scans. There were no significant between-group differences on any measure of upper extremity motor function at post-treatment.
The second fair quality RCT (Brogårdh & Sjülund, 2006) provided patients with chronic stroke with a 2-week CIMT program, then randomized patients to prolonged mitt use for a further 3 months, or no further treatment. Upper extremity motor function was measured at baseline and 2 weeks (post-treatment) using the modified Motor Assessment Scale (MAS) and the Sollerman Hand Function Test (SHFT). All participants demonstrated improved upper extremity motor function after 2 weeks of CIMT. After 3 months there were no significant differences in motor function between prolonged mitt use and no mitt use.
A 4-year follow-up to this study (Brogårdh et al., 2009a) found no significant change in SHFT scores when compared to baseline or post-treatment data. The MAS was not used on retesting.
A controlled clinical trial (Taub et al., 2006) assigned patients with chronic stroke to a CIMT group or a control group that received time-matched and interaction-matched physical, cognitive and relaxation exercises. Upper extremity motor function was measured at baseline and at 2 weeks (post-treatment) using the WMFT-PT and WMFT-FA. There was a significant between-group difference in WMFT–PT scores at post-treatment, in favour of CIMT compared to the control group. There were no significant between-group differences in WMFT-FA scores. Although measures were also taken at 3 follow-up intervals (4 weeks, 3 months, 2 years), statistical data for between-group differences were not reported at these time points.
A pre-post study without multiple baselines (Kunkel et al., 1999) examined the effects of CIMT in patients with chronic stroke. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and at 3 months (follow-up) using the ARAT and WMFT. There was a significant improvement in ARAT and WMFT scores at both time points.
Conclusion: There is conflicting evidence (level 4) regarding the effectiveness of CIMT compared to other interventions. Two high quality RCTs and one fair quality RCT reported that CIMT was not more effective than comparison interventions (Bobath Concept therapy, task-oriented training, rTMS+OT); in fact, 1 high quality RCT found that CIMT was less effective than rTMS+OT for improving upper extremity motor function. However, another high quality RCT and a controlled clinical trial reported that CIMT was more effective than comparison interventions (bimanual training, time-matched rehabilitation) on some measures of upper extremity motor function (ARAT and WMFT performance time). Further, 1 pre-post design study found significant improvement in upper extremity motor function following CIMT.
Note: A fair quality RCT found an improved upper extremity motor function after 2 weeks of CIMT, and note that prolonged mitt use following a CIMT program was not more effective than CIMT alone for improving upper extremity motor function in patients with chronic stroke.
Quality of movement (Upper extremity)
Not effective
1B
One high quality RCT (Huseyinsinoglu, Ozdincler, & Krespi, 2012) has investigated the effect of CIMT on quality of movement of the upper extremity in patients with chronic stroke. This high quality RCT randomized patients with chronic stroke to receive CIMT or Bobath Concept therapy. At post-treatment (2 weeks) there was no significant between-group difference in upper extremity quality of movement (Motor Evaluation Scale for Arm in Stroke Patients).
Conclusion: There is moderate (level 1b) evidence from 1 high quality RCT that CIMT is not more effective than a comparison intervention (Bobath Concept therapy) for improving upper extremity quality of movement among patients with chronic stroke.
Sensory discrimination
Not effective
2a
One fair quality RCT (Brogårdh & Sjülund, 2006) examined the effect of CIMT on sensory discrimination in patients with chronic stroke. This fair quality RCT provided patients with a 2-week CIMT program, then randomized patients to prolonged mitt use for a further 3 months, or no further treatment. There were no significant within-group differences in sensory discrimination (Two-Point Discrimination Test) following 2 weeks of CIMT. Further, there were no significant between-group differences in sensory discrimination at 3 months.
Conclusion: There is limited evidence (level 2a) from 1 fairquality RCTthat CIMT does not improve sensory discrimination in chronic stroke. Further, the fairquality RCTfound that prolonged mitt wear is not more effective than control therapy (i.e. no treatment) for improving sensory discrimination in patients with chronic stroke.
Chronic phase: mCIMT vs. control or alternative interventions
One high quality RCT (Barzel et al., 2015), 1 poor quality RCT (Kim et al., 2008) and 1 quasi-experimental study (Siebers & Skargren, 2010) investigated the effect of mCIMT on dexterity among patients with chronic stroke.
The high quality RCT (Barzel et al., 2015) randomized patients with chronic stroke to receive home-based mCIMT or conventional rehabilitation. Finger dexterity was measured at baseline, 4 weeks (post-treatment) and 6-month follow-up using the Nine Hole Peg Test. There was no significant between-group difference in finger dexterity any time point.
The poor quality RCT (Kim et al., 2008) randomized patients with chronic stroke to receive mCIMT or a control group (intervention not specified). Patients in the mCIMT group wore a modified opposition restriction orthosis (MORO) on the unaffected hand for at least 5 hours/day, 7 days a week. Dexterity was measured using the Purdue Pegboard Test at baseline and 8 weeks (post-treatment). There were no significant between-group differences in dexterity at post-treatment.
The quasi-experimental study (Siebers & Skargren, 2010) provided patients with chronic stroke with mCIMT that comprised restraint for 90% of waking hours and an individualized training program for 6 hours/weekday. Manual dexterity was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the Box and Block Test (BBT). There was a significant improvement in manual dexterity from baseline to post-treatment, but this did not remain significant at 6-month follow-up.
Note: As this study did not report between-group differences these results are not used to determine level of evidence below.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT and 1 poorquality RCT that mCIMT is not more effective than comparison interventions (conventional rehabilitation) for improving dexterity among patients with chronic stroke.
Note: One quasi-experimental study reported a significant improvement, in short term, in manual dexterity from baseline to post-treatment but as this study did not report between-group differences these results are not used to determine level of evidence.
Grip strength
Insufficient evidence
5
One quasi-experimental study (Siebers & Skargren, 2010) investigated the effect of mCIMT on grip strength among patients with chronic stroke. This quasi-experimental study provided patients with chronic stroke with mCIMT that comprised restraint for 90% of waking hours and an individualized training program for 6 hours/weekday. Grip strength was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the Grippit instrument. There was a significant improvement in grip strength from baseline to post-treatment and this persisted at 6-month follow-up.
Conclusion: There is insufficient evidence (level 5) regarding the effectiveness of mCIMT on grip strength compared to other interventions. However, 1 quasi-experimental study reported improved grip strength among patients with chronic stroke following mCIMT.
Functional independence and activities of daily living
Effective
1A
Four high quality RCTs (Lin et al., 2007, Wu et al., 2007c,, Lin et al., 2009b; Barzel et al., 2015) and 2 fair quality RCTs (Lin et al., 2008, Wu et al., 2012b) examined the effectiveness of mCIMT on functional independence in patients with chronic stroke.
The first high quality RCT (Lin et al., 2007) randomized patients with chronic stroke to either mCIMT or traditional rehabilitation. The mCIMT group had their unaffected hand restricted by a mitt for 6 hours per day and received intensive training on the affected arm for 2 hours per weekday. Functional independence was measured at baseline and 3 weeks (post-treatment) using the Functional Independence Measure (FIM). There were significant between-group differences in functional independence at post-treatment, in favour of mCIMT compared to traditional rehabilitation.
The second high quality RCT (Wu et al., 2007c) randomized patients with chronic stroke to either mCIMT or neurodevelopmental therapy. The mCIMT group wore a constraint on the less affected UE 6 hours a day while receiving 2 hours daily of intensive training. Functional independence was measured at baseline and 3 weeks (post-treatment) using the FIM. The mCIMT group had significantly greater gains in functional independence than the control group at post-treatment.
The third high quality RCT (Lin et al., 2009b) randomized patients with chronic stroke to receive mCIMT, bilateral arm training (BAT), or standard UE therapy (control). The mCIMT group received 2 hours per weekday of UE therapy for 12 days for 3 weeks while wearing a restraint on the unaffected UE for 6 hours per day. Functional independence was measured at baseline and 3 weeks (post-treatment) using the FIM. The mCIMT group showed significantly greater functional independence than the BAT group and the control group at post-treatment.
The fourth high quality RCT (Barzel et al., 2015) randomized patients with chronic stroke to receive home-based mCIMT or conventional rehabilitation. Functional independence was measured at baseline, 4 weeks (post-treatment) and 6-month follow-up using the Barthel Index (BI) and the Instrumental Activities of Daily Living (IADL). There were no significant between-group differences in functional independence on either measure at any time point.
The first fair quality RCT (Lin et al., 2008) randomized patients with chronic stroke to either mCIMT or traditional intervention. The mCIMT group wore restraints on the hand and wrist for 3 hours/weekday and received 2-hour training sessions each weekday. Functional independence was measured at baseline and 3 weeks (post-treatment) using the FIM and the Nottingham Extended Activities of Daily Living (NEADL). The mCIMT group showed significantly greater improvements in functional independence (FIM total, self-care and locomotion domains; NEADL mobility domain only) than the control group at post-treatment.
The second fair quality RCT (Wu et al., 2012b) randomized patients with chronic stroke to receive mCIMT, mCIMT with trunk restraint (mCIMT-TR) or conventional rehabilitation based on neurodevelopmental principles. Both mCIMT groups wore a mitt on the non-affected hand and wrist for 5 hours/day, the mCIMT-TR group wore a harness to restrain the trunk during rehabilitation sessions; all groups received their respective intervention for 2 hours/day, 5 days/week. Functional independence was measured at baseline and 3 weeks (post-treatment) using the Functional Activities Index (FAI). There were significant between-group differences in functional independence in favour of mCIMT compared to conventional rehabilitation (FAI total score only) at post-treatment.
Note: There were also significant between-group differences in favour of mCIMT-TR compared to conventional rehabilitation (FAI outdoor and total scores); there were no significant between-group differences on other measures of functional independence (FAI domestic chores, leisure/work subtests).
Conclusion: There is strong evidence (level 1a) from 3 high quality RCTs and 2 fair qualityRCTs that mCIMT is more effective than control therapies (e.g. conventional rehabilitation, neurodevelopmental therapy, bilateral arm training) for improving functional independence in patients with chronic stroke.
Note: However, one high quality RCT found that mCIMT was not more effective than conventional rehabilitation for improving functional independence among patients with chronic stroke. This high quality RCT used different measures of functional independence to the other RCTs reviewed above.
Kinematics (Upper extremity)
Effective
1a
Four high quality RCTs (Wu et al., 2011; Wu et al., 2007c; Lin et al., 2007; Wu et al., 2012a), 1 fair quality RCT (Wu et al., 2012b) and 2 pre-post studies (Caimmi et al., 2008; Richards et al., 2008) have investigated the effect of mCIMT on upper extremity kinematics in patients with chronic stroke.
The first high quality RCT (Wu et al., 2011) randomized patients with chronic stroke to receive mCIMT, bilateral arm therapy (BAT) or conventional therapy. mCIMT comprised use of a restrictive mitt for 6 hours/weekday. All groups received 2 hours of occupational therapy daily over the intervention period. Unilateral and bilateral reach kinematics were measured at baseline and 3 weeks (post-treatment) according to movement efficiency (normalized movement time – NMT), movement smoothness (normalized movement unit – NMU), peak velocity (PV) and movement strategies (percentage of movement time when peak velocity occurred – PPV). At post-treatment there was a significant between-group difference in unilateral and bilateral NMU in favour of the mCIMT group compared to the control group.
Note: There was also a significant between-group difference in unilateral and bilateral NMU and PV, in favour of the BAT group compared to the control group. There were no differences between groups for other kinematic measures.
The second high quality RCT (Wu et al., 2007c) randomized patients with chronic stroke to either mCIMT or neurodevelopmental therapy (NDT). The mCIMT group wore a constraint on the less affected UE 6 hours/ day while receiving 2 hours daily of intensive training. Kinematic measures were taken at baseline and 3 weeks (post-treatment). The mCIMT group showed more temporally and spatially efficient movement and more preplanned movement during bimanual tasks than the NDT group at post-treatment. During unilateral task performance, the mCIMT group produced more ballistic/preplanned reaching movement than the NDT group but did not produce significant between-group differences in temporal or spatial efficiency.
The third high quality RCT (Lin et al., 2007) randomized patients with chronic stroke to receive mCIMT or traditional rehabilitation. The mCIMT group wore a mitt for 6 hours/day and received intensive training on the affected arm for 2 hours/weekday. Kinematic measures were taken at baseline and 3 weeks (post-treatment) and included reaction time, NMT, PPV, NMU, maximum grip aperture, and percentage of movement time where maximum grip aperture occurs. At post-treatment, significant between-group differences were reported for reaction time and PPV, in favour of mCIMT compared to traditional rehabilitation.
The fourth high quality RCT (Wu et al., 2012a) randomized patients with chronic stroke to receive modified CIMT (mCIMT), modified CIMT with trunk restraint (mCIMT+TR) or conventional rehabilitation. The mCIMT groups received therapy for 2 hours/weekday and wore a mitt for 6 hours/day. Upper extremity kinematics were measured at baseline and 3 weeks (post-treatment) and measures included grasp, shoulder and elbow joint range and trunk movement. There were no significant differences in grasping, joint range and trunk movement during reach-to-grasp between mCIMT and conventional rehabilitation.
Note: There were significant differences in grasp kinematics in favour of mCIMT+TR compared to conventional rehabilitation (active shoulder movement, trunk movement), and in favour of mCIMT+TR compared to mCIMT (active shoulder movement, active elbow movement, trunk movement).
The fair quality RCT (Wu et al., 2012b) randomized patients with chronic stroke to receive modified constraint-induced movement therapy (mCIMT), mCIMT with trunk restraint (mCIMT-TR) or conventional rehabilitation based on neurodevelopmental principles. Both mCIMT groups wore a mitt on the non-affected hand and wrist for 5 hours/day and the mCIMT-TR group wore a harness to restrain the trunk during rehabilitation sessions; all groups received their respective intervention for 2 hours/day, 5 days/week. Kinematic measures including trunk slope (start, middle, end reach) and normalized shoulder and elbow flexion were taken at baseline and 3 weeks (post-treatment). There were no significant differences in kinematic measures at post-treatment between mCIMT and conventional rehabilitation.
Note: there were significant between-group differences in kinematics in favour of mCIMT-TR compared to conventional rehabilitation (trunk slope – start), and in favour of mCIMT-TR compared to mCIMT (normalized shoulder flexion).
The first pre-post study without multiple baselines (Caimmi et al., 2008) tested the suitability of a new method of kinematic analysis for evaluating the effects of mCIMT in patients with chronic stroke. Patients wore a splint on the less affected limb for approximately 80% of waking hours for 14 consecutive days and received physiotherapy and occupational therapy for 1 hour/weekday. Movement of the affected arm was measured using kinematic testing at baseline and 2 weeks (post-treatment). The mCIMT group demonstrated an improvement in the movement efficiency of the affected arm at post-treatment.
The second pre-post study without multiple baselines (Richards et al., 2008) examined the effect of CIMT protocols on UE kinematics in patients with chronic stroke. Subjects wore a mitten restraint during 90% of waking hours. Patients 1 and 2 received therapy 6 hours/day (CIMT) while patient 3 received therapy 3 hours/day (mCIMT). All participants demonstrated improved kinematic reaching values post-intervention, with no significant between-patient differences.
Conclusion: There is strong evidence (level 1a) from 3 high quality RCTs that mCIMT is more effective than control therapies (e.g. bilateral arm training, neurodevelopmental therapy and conventional rehabilitation) for improving upper extremity kinematic measures such as movement smoothness or efficiency, reaction time and reaching values in patients with chronic stroke. Further, 2 pre-post studies reported improvement in upper extremity kinematics following mCIMT.
Note: However, one high quality RCT and one fair quality RCT found that mCIMT was not more effective than conventional rehabilitation for improving upper extremity kinematics in patients with chronic stroke. Further, these RCTs reported that mCIMT with trunk restraint is more effective than mCIMT alone and conventional rehabilitation for improving some measures of upper extremity kinematics.
Motor activity (Upper extremity)
Effective
1A
Seven high quality RCTs (Page et al., 2004; Lin et al., 2007; Wu et al., 2007c; Lin et al., 2009b; Wu et al., 2011; Wu et al., 2012a; Barzel et al., 2015), 4 fair quality RCTs (Page et al., 2008; Lin et al., 2008; Lin et al., 2010; Wu et al., 2012b), 3 poor quality RCTs (Wu et al., 2010; Kim et al., 20080; Atteya, 2004) and 4 non-experimental studies (Barzel et al., 2009; Dettmers et al., 2005; Caimmi et al., 2008; Siebers & Skargren, 2010) have examined the effect of mCIMT on upper extremity (UE) motor activity in patients with chronic stroke.
The first high quality RCT (Page et al., 2004) randomized patients with chronic stroke to receive mCIMT + UE therapy, UE therapy alone, or no therapy. The mCIMT group wore a constraint on the affected extremity for 5 hours/day and received UE therapy for 30 minutes, 3 times/week. Upper extremity motor activity was measured at baseline and 10 weeks (post-treatment) using the Motor Activity Log – Amount of Use and – Quality of Movement (MAL-AOU, MAL-QOM). There were no significant differences between any group at post-treatment (10 weeks).
The second high quality RCT (Lin et al., 2007) randomized patients with chronic stroke to receive mCIMT or conventional rehabilitation. The mCIMT group wore a mitt for 6 hours/day and received intensive training on the affected arm for 2 hours/weekday. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL. There were significant between-group differences in motor activity at post-treatment, in favour of mCIMT compared to conventional rehabilitation.
The third high quality RCT (Wu et al., 2007c) randomized patients with chronic stroke to receive mCIMT or neurodevelopmental therapy (NDT). The mCIMT group wore a constraint on the less affected UE 6 hours/day and received 2 hours daily of intensive training. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. There was a significant between-group difference in motor activity improvement scores from baseline to post-treatment, in favour of mCIMT compared to NDT.
The fourth high quality RCT (Lin et al., 2009b) randomized patients with chronic stroke to receive mCIMT, bilateral arm training (BAT), or standard UE therapy. The mCIMT group received UE therapy 2 hours/weekday and wore a restraint on the unaffected UE for 6 hours/day. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. The mCIMT group showed significantly better MAL-AOU and MAL-QOM scores than the BAT group or the control group at post-treatment.
The fifth high quality RCT (Wu et al., 2011) randomized patients with chronic stroke to receive mCIMT, bilateral arm therapy (BAT) or conventional rehabilitation. The mCIMT group wore a restrictive mitt for 6 hours/day, 5 days/week and all groups received occupational therapy for 2 hours/day. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. There were significant between-group differences in MAL-AOU and MAL-QOM scores at post-treatment, in favour of the mCIMT group compared to BAT and conventional rehabilitation. There were no significant differences in motor activity between BAT and conventional rehabilitation.
The sixth high quality RCT (Wu et al., 2012a) randomized patients with chronic stroke to receive modified CIMT (mCIMT), modified CIMT with trunk restraint (mCIMT+TR) or conventional rehabilitation. The mCIMT groups wore a mitt on the unaffected hand for 6 hours/day and received intervention for 2 hours/weekday. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. There were no significant differences in MAL-AOU or MAL-QOM scores between any group pairing at post-treatment.
The seventh high quality RCT (Barzel et al., 2015) randomized patients with chronic stroke to receive home-based mCIMT or conventional rehabilitation. The mCIMT group wore a glove on the unaffected hand for 2-4 hours/day and performed exercises with the affected arm for 2 hours/weekday. Upper extremity motor activity was measured at baseline, 4 weeks (post-treatment) and 6-month follow-up using the MAL-AOU and MAL-QOM. There was a significant between-group difference in MAL-AOU and MAL-QOM scores at post-treatment and follow-up, in favour of home-based mCIMT compared with conventional rehabilitation.
The first fair quality RCT (Page et al., 2008) randomized patients to receive mCIMT, time-matched rehabilitation, or no treatment. Patients in the mCIMT group received functional practice sessions for 30 minutes/day and restricted us of the less-affected arm for 5 hours/weekday. Upper extremity motor activity was measured at baseline and 10 weeks (post-treatment) MAL-AOU and MAL-QOM. The mCIMT group demonstrated significantly improved MAL-AOU and MAL-QOM scores compared to the other treatment groups at post-treatment.
The second fair quality RCT (Lin et al., 2008) randomized patients with chronic stroke to receive mCIMT or conventional intervention. The mCIMT group wore restraints on the hand and wrist for 3 hours/weekday and received training sessions for 2 hours/weekday. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) MAL-AOU and MAL-QOM. There were no significant between-group differences in motor activity at post-treatment.
The third fair quality RCT (Lin et al., 2010) randomized patients with chronic stroke to receive mCIMT or conventional rehabilitation for the same intensity and duration. The mCIMT group wore a restrictive mitt 6 hours/weekday and received upper limb training 2 hours/weekday. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. There was a significant between-group difference in MAL-AOU and MAL-QOM scores at post-treatment, in favour of mCIMT compared to conventional rehabilitation.
The fourth fair quality RCT (Wu et al., 2012b) randomized patients with chronic stroke to receive modified constraint-induced movement therapy (mCIMT), mCIMT with trunk restraint (mCIMT-TR) or conventional rehabilitation based on neurodevelopmental principles. Both mCIMT groups wore a mitt on the non-affected hand and wrist for 5 hours/day and the mCIMT-TR group wore a harness to restrain the trunk during rehabilitation sessions; all groups received their respective intervention for 2 hours/weekday. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. There were significant between-group differences in MAL-AOU and MAL-QOM scores at post-treatment, in favour of mCIMT compared to conventional rehabilitation.
Note: There were also significant between-group differences in favour of mCIMT-TR compared to conventional rehabilitation (MAL-QOM only).
The first poor quality RCT (Wu et al., 2010) randomized patients with chronic stroke to receive mCIMT with intensive training of the upper extremity for 2 hours/weekday, or bilateral arm therapy training for the same frequency and duration. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. The mCIMT group demonstrated improved MAL-QOU and MAL-QOM scores at post-treatment.
Note: Statistical data and between-group differences were not reported; accordingly, this study is not included in determining level of evidence for the effectiveness of mCIMT compared to other interventions.
The second poor quality RCT (Kim et al., 2008) randomized patients to either mCIMT or a control group (intervention not specified). Patients in the CIMT group wore a modified opposition restriction orthosis (MORO) on the unaffected hand at least 5 hours/day. Upper extremity motor activity was measured at baseline and 8 weeks (post-treatment) using the MAL. Improved motor activity was seen post-treatment, although between-group statistical comparisons were not provided.
The third poor quality RCT (Atteya, 2004) randomized patients with chronic stroke to receive mCIMT, conventional rehabilitation or no therapy. Upper extremity motor activity was measured at baseline and 10 weeks (post-treatment) using the MAL-AOU and MAL-QOM. The mCIMT group demonstrated improved MAL-AOU and MAL-QOM scores at post-treatment compared to the control groups.
Note: Statistical data and between-group differences were not reported; accordingly, this study is not included in determining level of evidence for the effectiveness of mCIMT compared to other interventions.
A quasi-experimental study (Barzel et al., 2009) assigned patients with chronic stroke to receive either CIMT (physiotherapy 6 hours/weekday for 2 weeks and splint worn on unaffected hand for a target of 90% of waking hours) or a mCIMT home program (home-based training with a family member for 2 hours/weekday for 4 weeks and splint worn on the unaffected hand for a target of 60% of waking hours). Upper extremity motor activity was measured using the MAL-AOU and MAL-QOM at baseline, post-treatment and 6-month follow-up. There were no significant between-group differences in motor activity at any time point.
A pre-post study with multiple baselines (Dettmers et al., 2005) examined the effects of mCIMT on patients with chronic stroke. Patients underwent intensive motor training of the more-affected arm for 3 hours/day for 20 days. The unaffected arm was restrained for 9.3 hours/day to limit its use. Upper extremity motor activity was measured at baseline, 3 weeks (post-treatment) and at 6-month follow-up using the MAL. Significant improvements in motor activity MAL were found from pre- to post-treatment and were retained at follow-up (6 months).
A pre-post study without multiple baselines (Caimmi et al., 2008) tested the suitability of a new method of kinematic analysis for evaluating the effects of mCIMT in patients with chronic stroke. Patients wore a splint on the less affected limb for approximately 80% of waking hours for 14 consecutive days and received 1 hour of physiotherapy and occupational therapy every weekday. Upper extremity motor activity was measured at baseline and 2 weeks (post-treatment) using the MAL. The mCIMT group demonstrated significantly improved motor activity at post-treatment.
A quasi-experimental study (Siebers & Skargren, 2010) provided patients with chronic stroke with mCIMT that comprised restraint for 90% of waking hours and an individualized training program for 6 hours/weekday. Upper extremity motor activity was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the Motor Activity Log – Quality of Movement (MAL-QOM). There was a significant improvement in upper extremity motor activity from baseline to post-treatment but this did not remain significant at 6-month follow-up.
Conclusion: There is strong evidence (level 1a) from 5 high quality RCTs and 3 fair quality RCTs that mCIMT is more effective than other therapies (conventional rehabilitation, neurodevelopmental therapy, bilateral arm therapy or no treatment) for improving upper extremity motor activity in patients with chronic stroke. Further, 3 poor quality RCTs and 3 non-experimental studies found improved motor activity following mCIMT.
Note: However, 2 high quality RCTs and 1 fair quality RCT found that mCIMT was not more effective than control therapies (e.g. conventional rehabilitation or no treatment) for improving upper extremity motor activity in patients with chronic stroke. Also, 1 non experimental study found no improvement in motor activity of the upper extremity after mCIMT.
Note: One of the high quality RCT found that mCIMT alone is not more effective than mCIMT with trunk restraint for improving upper extremity motor activity in patients with chronic stroke and 1 fair quality RCT found that mCIMT alone is as effective as mCIMT with trunk restraint for improving motor activity of the upper extremity, both compared to conventional rehabilitation.
Motor function (Upper extremity)
Effective
1a
Five high quality RCTs (Page et al., 2004; Lin et al., 2009b; Wu et al., 2011; Barzel et al., 2015; Wu et al., 2012a), 5 quality RCTs (Page et al., 2008; Lin et al., 2008; Lin et al., 2010; Hayner, Gibson & Giles, 2010; Wu et al., 2012b), 3 poor quality RCTs (Wu et al., 2010; Kim et al., 2008; Atteya, 2004) and 3 non-experimental studies (Caimmi et al., 2008; Dettmers et al., 2005; Siebers & Skargren, 2010) have examined the effects of mCIMT on upper extremity (UE) motor function in patients with chronic stroke.
The first high quality RCT (Page et al., 2004) randomized patients with chronic stroke to receive mCIMT and UE therapy, conventional UE therapy alone, or no therapy. The mCIMT group wore a constraint on the affected extremity for 5 hours/day and received 30 minutes of UE therapy 3 times/week. Upper extremity motor function was measured at baseline and 10 weeks (post-treatment) using the Fugl-Meyer Assessment (FMA) and the Action Research Arm Test (ARAT). There were significant between-group differences at post-treatment in favour of mCIMT compared to no therapy (FMA, ARAT), and in favour of mCIMT compared to conventional rehabilitation (FMA only).
The second high quality RCT (Lin et al., 2009b) randomized patients with chronic stroke to receive mCIMT, bilateral arm training (BAT), or standard UE therapy. The mCIMT group wore a restraint on the unaffected UE for 6 hours/day and received UE therapy for 2 hours/weekday. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the FMA. At post-treatment there was a significant between-group difference on measures of upper extremity motor function (FMA overall score and distal score) in favour of mCIMT compared to standard UE therapy.
Note: At post-treatment there was also a significant difference in motor function (FMA proximal score only) in favour of the BAT group compared to the mCIMT group.
The third high quality RCT (Wu et al., 2011) randomized patients with chronic stroke to receive mCIMT, bilateral arm therapy (BAT) or conventional therapy. mCIMT comprised use of a restrictive mitt for 6 hours/weekday and all groups received occupational therapy for 2 hours/weekday. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the Wolf Motor Function Test Performance Time (WMFT-PT), Functional Ability (WMFT-FA) and Strength scores. There was a significant between-group difference in WMFT–PT and WMFT-FA scores at post-treatment, in favour of the mCIMT group compared to conventional therapy. There were no significant differences in motor function between the mCIMT and BAT groups, or between the BAT and control groups.
The fourth high quality RCT (Barzel et al., 2015) randomly assigned patients with chronic stroke to receive home-based mCIMT or conventional rehabilitation. The mCIMT group wore a glove on the unaffected hand for 2-4 hours/day and performced exercises with the affected arm for 2 hours/weekday. Upper extremity motor function was measured at baseline, 4 weeks (post-treatment) and 6-month follow-up using the WMFT-PT and WMFT-FA. There was no significant between-group difference in motor function at any time point.
The fifth high quality RCT (Wu et al., 2012a) randomly assigned patients with chronic stroke to receive modified CIMT (mCIMT), modified CIMT with trunk restraint (mCIMT+TR) or conventional rehabilitation. The mCIMT groups wore a mitt on the unaffected hand for 6 hours/day and received intervention for 2 hours/weekday. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the FMA-UE proximal, distal and total scores. There were no significant differences in upper extremity motor function between mCIMT and conventional rehabilitation.
Note: There were no significant differences in upper extremity motor function between mCIMT and mCIMT+TR; there were significant between-group differences in FMA-UE distal and total scores in favour of mCIMT+TR compared to conventional rehabilitation.
The first fair quality RCT (Page et al., 2008) randomized patients with chronic stroke to receive mCIMT, time-matched rehabilitation, or no treatment. The mCIMT group received functional practice sessions for 30 minutes/weekday and restrained the less-affected arm for 5 hours/weekday. Motor function was measured at baseline and 10 weeks (post-treatment) using the FMA and ARAT. The mCIMT group showed significant improvements in motor function (ARAT only) compared to the other 2 groups at post-treatment.
The second fair quality RCT (Lin et al., 2008) randomized patients with chronic stroke to receive mCIMT or traditional intervention. The mCIMT group wore a restraint for 3 hours/weekday and training sessions for 2 hours/weekday. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the FMA. There was a significant between-group difference in motor function (FMA improvement scores) at post-treatment, in favour of mCIMT compared to traditional intervention.
The third fair quality RCT (Lin et al., 2010) randomized patients with chronic stroke to receive either mCIMT or conventional rehabilitation for the same intensity and duration. mCIMT comprised use of a restrictive mitt 6 hours/weekday and upper limb training 2 hours/weekday, 5 days/week. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the FMA upper limb subscale. The mCIMT group demonstrated a significantly greater improvement in motor function than the control group at post-treatment.
The fourth fair quality RCT (Hayner, Gibson & Giles, 2010) randomized patients with chronic stroke to receive mCIMT or bilateral upper extremity training. The mCIMT group wore a restraining mitt 6 hours/day for 10 days. Upper extremity motor function was measured using the WMFT at baseline, 10 days (post-treatment) and at 6-month follow-up. There were no significant between-group differences in motor function at any time point.
Note: A comparison of ‘less impaired’ vs. ‘more impaired’ patients revealed a significant between-group difference in motor function (WMFT), in favour of ‘less impaired’ patients, regardless of intervention group.
The fifth fair quality RCT (Wu et al., 2012b) randomized patients with chronic stroke to receive modified constraint-induced movement therapy (mCIMT), mCIMT with trunk restraint (mCIMT-TR) or conventional rehabilitation based on neurodevelopmental principles. Both mCIMT groups wore a mitt on the non-affected hand and wrist for 5 hours/day and the mCIMT-TR group wore a harness to restrain the trunk during rehabilitation sessions; all groups received their respective intervention for 2 hours/weekday. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the ARAT. At post-treatment there were significant between-group differences in favour of mCIMT compared to conventional rehabilitation (ARAT gross and total scores only).
Note: At post-treatment there was also a significant between-group difference in in favour of mCIMT-TR compared to mCIMT (ARAT grip scores), and in favour of mCIMT-TR compared to conventional rehabilitation (ARAT grip, pinch, gross and total scores).
The first poor quality RCT (Wu et al., 2010) randomized patients with chronic stroke to receive mCIMT for 2 hours/weekday, or bilateral arm therapy training for the same frequency and duration. Upper extremity motor function was measured at baseline and post-treatment (3 weeks) using the ARAT and FMA upper extremity subscale. Patients from both groups demonstrated improved scores on measures of motor function at post-treatment.
Note: Statistical data and between-group differences were not reported; accordingly, this study is not included in determining level of evidence for the effectiveness of mCIMT compared to other interventions.
The second poor quality RCT (Kim et al., 2008) randomized patients with chronic stroke to a mCIMT group or a control group (intervention not specified). Patients in the mCIMT group wore a modified opposition restriction orthosis (MORO) on the unaffected hand at least 5 hours/day. Upper extremity motor function was measured at baseline and 8 weeks (post-treatment) using the Manual Function Test. There were no significant between-group differences in motor function at post-treatment.
The third poor quality RCT (Atteya, 2004) randomized patients with subacute stroke to receive mCIMT, conventional rehabilitation or no therapy. Upper extremity motor function was measured at baseline and 10 weeks (post-treatment) using the FMA, WMFT and ARAT. The mCIMT group demonstrated improved scores on all measures of motor function at post-treatment.
Note: Statistical data and between-group differences were not reported; accordingly, this study is not included in determining level of evidence for the effectiveness of mCIMT compared to other interventions.
A pre-post study without multiple baselines (Caimmi et al., 2008) tested the suitability of a new method of kinematic analysis for evaluating the effects of mCIMT in patients with chronic stroke. The patients wore a splint on the less affected limb for approximately 80% of waking hours for 14 consecutive days and received 1 hour of physiotherapy and occupational therapy every weekday. Motor function was assessed at baseline and 2 weeks (post-treatment) using the WMFT and the Motricity Index upper extremity subtests. At post treatment the patients demonstrated significantly improved within-subject pre-post scores on the WMFT but not on the Motricity Index.
A pre-post study with multiple baselines (Dettmers et al., 2005) examined the effects of mCIMT in patients with chronic stroke. Patients received intensive motor training of the more-affected arm for 3 hours/day for 20 days. The unaffected arm was restrained for 9.3 hours daily to limit its use. Upper extremity motor function was measured at baseline, 3 weeks (post-treatment) and at 6-month follow-up using the WMFT, Frenchay Arm Test, and grip strength. There were significant improvements on all measures of motor function from pre- to post-treatment, and results were retained at 6-month follow-up.
A quasi-experimental study (Siebers & Skargren, 2010) provided patients with chronic stroke with mCIMT that comprised restraint for 90% of waking hours and an individualized training program for 6 hours/weekday. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the Sollerman hand function test. There was a significant improvement in motor function from baseline to post-treatment and this persisted at 6-month follow-up.
Conclusion: There is strong evidence (level 1a) from 3 high quality RCTs and 4 fair quality RCTs that mCIMT is more effective than control interventions (conventional rehabilitation, bilateral arm training or no treatment) for improving upper extremity motor function in patients with chronic stroke. Further, one poor quality RCTs and 3 non-randomized studies also found significant improvement in upper extremity motor function following mCIMT.
Note: However, 2 high quality RCTs and 1 fair quality RCT reported that mCIMT is not more effective than comparison interventions (conventional rehabilitation, mCIMT with trunk restraint or bilateral arm training) for improving upper extremity motor function among patients with chronic stroke. Further, two poor quality RCTs did not find any significant between group difference when comparing mCIMT to a control intervention.
Note: Interestingly, one high quality RCT found a significant between group difference in favour of bilateral arm training when compared to mCIMT for one subscale of upper extremity motor function.
Range of motion
Insufficient evidence
5
One non-randomized study (Siebers & Skargren, 2010) has investigated the effect of mCIMT on range of motion among patients with chronic stroke. This quasi-experimental study provided patients with chronic stroke with mCIMT that comprised restraint for 90% of waking hours and an individualized training program for 6 hours/weekday. Active range of motion on elbow extension and wrist dorsiflexion was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using a goniometer. There was a significant improvement in elbow extension from baseline to post-treatment and this persisted at 6-month follow-up; there was a significant improvement in wrist dorsiflexion from baseline to post-treatment, but this did not remain significant at 6-month follow-up.
Conclusion: There is insufficient evidence (level 5) regarding the effect of mCIMT on upper extremity range of motion when compared with other interventions. However, 1 non-randomized study reported improved active range of motion among patients with chronic stroke following mCIMT.
Spasticity
Insufficient evidence
5
One non-randomized study (Siebers & Skargren, 2010) has investigated the effect of mCIMT on spasticity among patients with chronic stroke. This quasi-experimental study provided patients with chronic stroke with mCIMT that comprised restraint for 90% of waking hours and an individualized training program for 6 hours/weekday. Spasticity at the elbow and wrist was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the Modified Ashworth Scale. There was a significant improvement in wrist spasticity from baseline to post-treatment and this persisted at 6-month follow-up; there was a significant improvement in elbow spasticity from baseline to follow-up.
Conclusion: There is insufficient evidence (level 5) regarding the effect of mCIMT on upper extremity spasticity when compared with other interventions. However, 1 non-randomized study reported improved upper extremity spasticity among patients with chronic stroke following mCIMT.
Stroke outcomes
Effective
1B
Two high quality RCTs (Lin et al., 2009b; Barzel et al., 2015), 1 fair quality RCT (Wu et al., 2012b), and 1 pre-post study with multiple baselines (Dettmers et al., 2005) examined the effects of mCIMT on stroke outcomes in patients with chronic stroke.
The first high quality RCT (Lin et al., 2009b) randomized patients with chronic stroke to receive mCIMT, bilateral arm training (BAT), or standard UE therapy (control). The mCIMT group received UE therapy for 2 hours/weekday and wore a restraint on the unaffected UE for 6 hours/day. Stroke outcomes were measured at baseline and 3 weeks (post-treatment) using the Stroke Impact Scale (SIS). There were significant between-group differences in stroke outcomes at post-treatment, in favour of mCIMT compared to standard UE therapy (SIS overall score, ADL/IADL and hand function domains), and in favour of mCIMT compared to BAT (SIS overall score, ADL/IADL and social participation domains).
The second high quality RCT (Barzel et al., 2015) randomized patients with chronic stroke to receive home-based mCIMT or conventional rehabilitation. Stroke outcomes were measured at baseline, 4 weeks (post-treatment) and 6-month follow-up using the SIS. There was no significant between-group difference in hand function (SIS – Hand function) at post-treatment (4 weeks) or follow-up (6 months post-treatment).
The fair quality RCT (Wu et al., 2012b) randomized patients with chronic stroke to receive modified constraint-induced movement therapy (mCIMT), mCIMT with trunk restraint (mCIMT-TR) or conventional rehabilitation based on neurodevelopmental principles. Both mCIMT groups wore a mitt on the non-affected hand and wrist for 5 hours/day and the mCIMT-TR group wore a harness to restrain the trunk during rehabilitation sessions; all groups received their respective intervention for 2 hours/weekday. Stroke outcomes were measured at baseline and 3 weeks (post-treatment) using the SIS. At post-treatment there were significant between-group differences in stroke outcomes in favour of mCIMT compared to conventional rehabilitation (SIS strength and hand function scores); and in favour of mCIMT compared to mCIMT-TR (SIS strength scores).
Note: There were also significant between-group differences in favour of mCIMT-TR compared to conventional rehabilitation (SIS hand function scores only).
The pre-post study with multiple baselines (Dettmers et al., 2005) examined the effects of CIMT on patients with chronic stroke. Patients underwent intensive motor training of the more-affected arm for 3 hours a day for 20 days. The unaffected arm was restrained for 9 hours daily to limit its use. Stroke outcomes were assessed at baseline and 3 weeks (post-treatment) by the SIS. Participants displayed improved stroke outcomes (SIS physical, social participation and communication domains).
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT and 1 fair qualityRCT that mCIMT is more effective than control therapies (e.g. bilateral arm training, conventional rehabilitation or mCIMT with trunk restraint) for improving stroke outcomes in patients with chronic stroke. One non-experimental study also found improvements following mCIMT.
Note: One high quality RCT found that home-based mCIMT was not more effective than conventional rehabilitation for improving stroke outcomes, where the only measure used was the SIS hand function domain.
Chronic phase: CIMT vs. mCIMT
Motor activity (Upper extremity)
Not effective
1B
One high quality RCT(Sterr et al., 2002) and 2 non-experimental studies (Barzel et al., 2009; Richards et al., 2008) have compared the effect of CIMT and mCIMT on upper extremity motor activity in patients with chronic stroke.
The high quality RCT (Sterr et al., 2002) randomized patients with chronic stroke to receive CIMT (6 hours of UE therapy) or mCIMT (3 hours of UE therapy). Both groups wore a restraining splint or mitt for 90% of waking hours. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and 4-week follow-up using the Motor Activity Log – Amout of Use and – Quality of Movement (MAL-AOU, MAL-QOM). Both groups demonstrated significantly improve MAL-AOU and MAL-QOM scores at post-treatment. Comparison of scores from baseline to 4-week follow-up revealed a greater improvement in motor activity in favour of CIMT compared to mCIMT.
A quasi-experimental study (Barzel et al., 2009) assigned patients with chronic stroke to receive either CIMT (physiotherapy 6 hours/weekday for 2 weeks and splint worn on unaffected hand for a target of 90% of waking hours) or a mCIMT home program (home-based training with a family member for 2 hours/weekday for 4 weeks and splint worn on the unaffected hand for a target of 60% of waking hours). Upper extremity motor activity was measured using the MAL-AOU and MAL-QOM at baseline, post-treatment and 6-month follow-up. There were no significant between-group differences in motor activity at any time point.
A pre-post study without multiple baselines (Richards et al., 2008) examined the effect of CIMT protocols on motor activity in patients with chronic stroke. Subjects wore a mitten restraint during 90% of waking hours. Patients 1 and 2 received therapy 6 hours/day (CIMT) while patient 3 received therapy 3 hours/day (mCIMT). At post-treatment all patients demonstrated improved motor activity (MAL-AOU, MAL-QOM).
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that CIMT is not more effective than mCIMT for improving upper extremity motor activity among patients with chronic stroke in the short-term. Further, 2 non-experimental studies found no significant difference on motor activity when comparing CIMT with mCIMT.
Note: However, the high quality RCT found that CIMT was more effective than mCIMT four weeks post-treatment.
Motor function (Upper extremity)
Not effective
1B
One high quality RCT (Sterr et al., 2002) and 2 non-experimental studies (Barzel et al., 2009; Richards et al., 2008) have compared the effects of CIMT and mCIMT on motor function in patients with chronic stroke.
The high quality RCT (Sterr et al., 2002) randomized patients with chronic stroke to receive CIMT (6 hours of UE therapy), or mCIMT (3 hours of UE therapy). Both groups were required to wear a restraining splint or mitt for 90% of waking hours. Upper extremity motor function was measured at baseline and 2 weeks (post-treatment) using the Wolf Motor Function Test (WMFT). There were no significant between-group differences in motor function at post-treatment.
A quasi-experimental study (Barzel et al., 2009) assigned patients with chronic stroke to receive traditional CIMT (restraint for a target of 90% of waking hours and physiotherapy for 6 hours/weekday for 2 weeks) or a mCIMT home program (restraint for a target of 60% of waking hours and home-based training with a family member for 2 hours/weekday for 4 weeks). Upper extremity motor function was measured at baseline, 2 weeks and 6-month follow-up using the WMFT – Performance Time and – Functional Ability scales. There were no significant between-group differences in motor function at post-treatment or follow-up
A pre-post study without multiple baselines (Richards et al., 2008) examined the effect of CIMT protocols on UE function in patients with chronic stroke. Subjects wore a mitten restraint during 90% of waking hours. Patients 1 and 2 received therapy 6 hours/day (CIMT) while patient 3 received therapy 3 hours/day (mCIMT). At post-treatment all patients demonstrated improved motor function (FMA, WMFT).
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that CIMT is not more effective than mCIMT for improving upper extremity motor function in patients with chronic stroke. Further, 2 non-experimental studies found no significant difference on motor function when comparing CIMT with mCIMT.
Phase of stroke recovery not specific to one period: CIMT vs. control or alternative interventions
Functional independence
Not effective
1b
One high quality RCT (Dahl et al., 2008) examined the effects of CIMT on functional independence in patients with stroke. This high quality RCT randomized patients between 1 and 92 months post stroke to CIMT plus conventional rehabilitation or conventional rehabilitation alone. Functional independence was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the Functional Independence Measure (FIM). There were no significant between-group differences in functional independence at any time point.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that CIMT is not more effective than conventional rehabilitation in improving functional independence in patients with stroke.
Motor activity (Upper extremity)
Conflicting
4
Motor function (Upper extremity)
Conflicting
4
Three high quality RCTs (Wolf et al., 2006, – Wolf et al., 2008, 2 year follow-up of 2006 study participants; Wolf et al., 2010, crossover follow-up of 2006 study participants –; Dahl et al., 2008; Underwood et al., 2006), 1 fair quality RCT (Alberts et al., 2004) and 1 poor quality RCT (Lang, Thompson & Wolf, 2013) examined the effects of CIMT on upper extremity motor function in patients with stroke.
The first high quality RCT (Wolf et al., 2006) randomized patients between 3 and 9 months post stroke to CIMT with intensive UE therapy or usual care. Upper extremity motor function was measured at baseline, 2 weeks (pst-treatment) and 4-month, 8-month and 12-month follow-up using the Wolf Motor Function Test Performance Time (WMFT-PT), Functional Ability (WMFT-FA), grip strength and weight subtests. The CIMT group had significant improvements in WMFT-PT and WMFT-FA scores at post-treatment compared to the usual care group; differences in WMFT-PT scores remained significant at 4-month, 8-month and 12-month follow-up. There was no significant between-group difference in WMFT grip strength or weight scores at post-treatment, but the CIMT group showed significantly larger improvement in scores on these items than the usual care group at 12-month follow-up.
In the follow-up study of the study group described above (Wolf et al., 2008), differences were found to remain significant at 24 months, with further gains in the WMFT grip strength and weight items.
Further to the 2006 study, a crossover RCT (Wolf et al., 2010) was conducted to compare the effects of early CIMT (CIMT provided 3-9 months after stroke) and delayed CIMT (CIMT provided 15-24 months after stroke) among patients with subacute and chronic stroke. Comparison of scores at 12 months revealed a significant between-group difference in motor function (WMFT-PT, WMFT-FA) in favour of the group who had received CIMT compared to those who had not yet commenced CIMT. These results did not remain significant at 24 months, by which stage both groups had received CIMT.
The second high quality RCT (Dahl et al., 2008) randomized patients between 1 and 92 months post stroke to CIMT plus traditional rehabilitation or traditional rehabilitation alone. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and 6-month follow-up using the WMFT-PT and WMFT-FA subtests. There was a significant between-group difference post treatment in WMFT-PT and WMFT-FA scores at post-treatment, in favour of the CIMT group. However, differences were no longer significant at follow-up.
The third high quality RCT (Underwood et al., 2006) randomized participants from the EXCITE trial with subacute or chronic stroke to receive CIMT or no CIMT (CIMT delayed to 1 year after enrollment in the study). Upper extremity motor function was measured at baseline and 2 weeks (post-treatment) using the WMFT. There were no significant between-group differences in upper extremity motor function at post-treatment between the group who had received CIMT and those who had not yet commenced CIMT.
The fair quality RCT (Alberts et al., 2004) randomized patients from the EXCITE trial with subacute or chronic stroke to receive CIMT or no CIMT (CIMT delayed to 1 year after baseline assessment). Upper extremity function was measured at baseline and 2 weeks (post-treatment) using the WMFT, Fugl-Meyer Assessment (FMA) and a key turning activity. There was a significant difference in maximum precision grip force in favour of the group that had received CIMT compared to the group that had not yet commenced CIMT. There were no other significant differences between groups.
The poor quality RCT (Lang, Thompson & Wolf, 2013) randomized participants from the EXCITE Trial with subacute to chronic stroke to receive immediate CIMT or delayed CIMT. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and 12-month follow-up using the WMFT. There was a significant between-group difference in only one measure of upper extremity motor function (WMFT lift pencil task) at post-treatment, in favour of immediate compared to delayed CIMT. There were no significant between-group differences at follow-up.
Conclusion: There is conflicting evidence (level 4) regarding the effectiveness of CIMT for improving motor function in stroke. While 2 high quality RCTs found that CIMT was more effective than control therapies (e.g. conventional rehabilitation, no-CIMT*), 1 high quality RCT, 1 fair quality RCT and 1 poor quality RCT found that CIMT was not more effective than no-CIMT*.
* Several studies used ‘delayed CIMT’ control groups, which received CIMT approximately 12 months later than the intervention groups. Given that the ‘delayed CIMT’ groups had not yet received CIMT at the point of comparison, these control groups are referred to as ‘no CIMT’ for the purpose of comparison of effectiveness.
NOTE: Results from 1 high quality RCT show that providing CIMT 12 months post-stroke can still benefit upper extremity function.
One high quality RCT (Underwood et al., 2006) examined the effects of CIMT on pain in patients with stroke. This high quality RCT randomized participants from the EXCITE trial with subacute or chronic stroke to receive immediate CIMT or delayed CIMT (1 year after enrollment in the study). Pain was measured at baseline and 2 weeks (post-treatment) using the Fugl-Meyer Assessment joint pain subtest and a non-standardized scale of pain. There were no significant between-group differences in pain at post-treatment.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that CIMT does not cause more pain than a control therapy (no-CIMT*) in subacute or chronic stroke.
* The study used a ‘delayed CIMT’ control group, which received CIMT approximately 12 months later than the intervention group. Given that the control group had not yet received CIMT at 2 weeks, the control group is considered to have received ‘no CIMT’.
Stroke outcomes
Conflicting
4
Two high quality RCTs (Wolf et al., 2006, – Wolf et al., 2008, follow-up of 2006 study group, Wolf et al., 2010, crossover follow-up of 2006 study group –; Dahl et al., 2008) examined the effects of CIMT on stroke outcomes in patients with stroke.
The first high quality RCT (Wolf et al., 2006) randomized patients between 3 and 9 months post stroke to CIMT plus intensive UE therapy or usual care. Stroke outcomes were measured at baseline and at 4-month and 12-month follow-up using the Stroke Impact Scale (SIS – hand function, physical function subtests). There were no significant between-group differences in SIS physical function scores at either follow-up time point. The CIMT recipients displayed larger gains than the control group on the SIS hand function domain at both follow-up time points.
A follow-up study (Wolf et al., 2008), found that differences remained significant at 24 months.
Further to the 2006 study, a crossover RCT (Wolf et al., 2010) was conducted to compare the effects of early CIMT (CIMT provided 3-9 months after stroke) and delayed CIMT (CIMT provided 15-24 months after stroke) among patients with subacute and chronic stroke. At 12 months there were significant between-group differences in stroke outcomes (SIS hand function, ADL/IADL domains), in favour of the group who received CIMT compared to those who had not yet received CIMT. At 24 months, by which stage both groups had received CIMT, there were significant between-group differences in stroke outcomes (SIS hand function, ADL/IADL and communication domains), in favour of the early CIMT compared to the delayed CIMT group.
The second high quality RCT (Dahl et al., 2008) randomized patients between 1 and 92 months post stroke to CIMT plus traditional rehabilitation or traditional rehabilitation alone. Stroke outcomes were measured at baseline, 2 weeks (post-treatment) and 6-month follow-up. There were no significant between-group differences in SIS scores at any time point.
Conclusion: There is conflicting evidence (level 4) between 1 high quality RCT that found CIMT is not more effective than conventional rehabilitation for improving stroke outcomes, and 1 highquality RCTthat found CIMT is more effective than control therapies (usual care, no-CIMT*) for improving stroke outcomes.
* One study used ‘delayed CIMT’ control groups, which received CIMT approximately 12 months later than the intervention groups. Given that the ‘delayed CIMT’ group had not yet received CIMT at 12 months, at this time point the control group is considered to have received ‘no CIMT’. Comparison at 24 months, by which time both groups had received CIMT, is considered ‘early CIMT’ vs. ‘delayed CIMT’.
NOTE: There is evidence from 1 high quality RCT that providing CIMT in the subacute phase of stroke is more effective than delaying CIMT for 12 months after stroke for improving stroke outcomes.
Phase of stroke recovery not specific to one period: mCIMT vs. control or alternative interventions
Functional independence
Effective
1a
Two high quality RCTs (Wu et al., 2007b; Batool et al., 2015) examined the effects of mCIMT on functional independence in patients with stroke.
The first high quality RCT (Wu et al., 2007b) randomized patients between 3 weeks and 35 months since stroke to receive mCIMT or neurodevelopmental therapy (NDT). Functional independence was measured at baseline and 3 weeks (post-treatment) using the Functional Independence Measure (FIM). There were significant between-group differences in functional independence at post-treatment, in favour of mCIMT compared to NDT.
The second high quality RCT (Batool et al., 2015) randomized patients who were 2 weeks to 3 months post-stroke to receive mCIMT or a motor relearning program. The mCIMT group wore a mitt during 2-hour training sessions, 6 times/week. Functional independence was measured at baseline and 3 weeks (post-treatment) using the Functional Independence Measure (FIM) – eating, grooming, bathing, dressing upper body, dressing lower body and total scores. There were significant between-group differences on all FIM domains except FIM – dressing upper body at post-treatment, in favour of mCIMT compared to the motor relearning program.
Conclusion: There is strong evidence (level 1a) from 2 high quality RCTs that mCIMT is more effective than control therapy (e.g. neurodevelopmental therapy, motor relearning program) for improving functional independence following stroke.
Kinematics (Upper extremity)
Effective
1B
One high quality RCT (Wu et al., 2007a) examined the effects of mCIMT on upper extremity kinematics in patients with stroke. This high quality RCT randomized patients between 3 weeks and 35 months since stroke to either mCIMT or neurodevelopmental therapy. Patients in the mCIMT group received intensive functional training of the affected UE for 2 hours/day and wore a restraining mitt on the less-affected UE for 6 hours /day. Kinematic measures were taken at baseline and 3 weeks (post-treatment). At post-treatment the mCIMT group had significantly better strategies for reaching control than the control group on the kinematic movement analysis, specifically on reaction time, movement time, total upper extremity displacement, and smoothness of movement units. There was no significant between-group difference for the kinematic variable peak velocity at post-treatment.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is more effective than control therapy (e.g. neurodevelopmental therapy) for improving upper extremity kinematics in patients with stroke.
Motor activity (Upper extremity)
Conflicting
4
Five high quality RCTs (Wu et al., 2007a, Wu et al., 2007b; Abu Tariah et al., 2010; Khan et al., 2011; Smania et al., 2012) examined the effect of mCIMT on motor activity in patients with stroke.
The first high quality RCT (Wu et al., 2007a) randomized patients between 3 weeks and 35 months since stroke to receive mCIMT or neurodevelopmental therapy. The mCIMT group received intensive functional training of the affected UE for 2 hours/day and wore a restraining mitt on the less-affected UE 6 hours/day. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the Motor Activity Log – Amount of Use and – Quality of Movement (MAL-AOU, MAL-QOM). There were significant between-group differences in MAL-AOU and MAL-QOM scores at post-treatment, in favour of mCIMT compared to NDT.
The second high quality RCT (Wu et al., 2007b) randomized patients between 3 weeks and 35 months since stroke to receive mCIMT or NDT. The mCIMT group received intensive functional training of the affected UE for 2 hours/day and wore a restraining mitt on the less-affected UE 6 hours/day. Upper extremity motor activity was measured at baseline and 3 weeks (post-treatment) using the MAL-AOU and MAL-QOM. There were significant between-group differences in MAL-AOU and MAL-QOM scores at post-treatment, in favour of mCIMT compared to NDT.
The third high quality RCT (Abu Tariah et al., 2010) randomized patients with subacute or chronic stroke to receive mCIMT or NDT. Upper extremity motor activity was measured at baseline, 2 months (post-treatment) and 6-month follow-up using the MAL-AOU and MAL-QOM. There were no significant differences in motor activity at any time point.
The fourth high quality RCT (Khan et al., 2011) randomized patients with subacute to chronic stroke to receive mCIMT, conventional neurological therapy or therapeutic climbing. The mCIMT group received physiotherapy and occupational therapy for 5 hours/week while wearing a mitt, and an additional 5 hours/week of self-training of repetitive task-oriented activities. Upper extremity motor activity was measured at baseline, 5 weeks (post-treatment) and 6-month follow-up using the MAL-AOU and MAL-QOM. There were no significant between-group differences in upper extremity motor activity at any time point.
The fifth high quality RCT (Smania et al., 2012) randomized patients with subacute to chronic stroke to receive mCIMT or conventional rehabilitation. The mCIMT group wore a splint on the unaffected arm for 12 or more hours/day and received rehabilitation two hours/weekday. Upper extremity motor activity was measured at baseline, 2 weeks (post-treatment) and 3-month follow-up using the MAL-AOU and MAL-QOM. There were significant between-group differences in MAL-AOU and MAL-QOM scores at post-treatment and follow-up, in favour of mCIMT compared to conventional rehabilitation.
Conclusion: There is conflicting evidence (level 4) from 5 high quality RCTs regarding the effectiveness of mCIMT in comparison to other interventions (neurodevelopmental therapy, conventional rehabilitation) for improving upper extremity motor activity following stroke. While 3 high quality RCTs reported significant difference in favour of mCIMT, 2 high quality RCTs found no significant difference in upper extremity motor activity following mCIMT compared to other therapies (neurodevelopmental therapy, conventional neurological therapy, therapeutic climbing). Interestingly, these studies provided mCIMT over a longer time period (5 weeks, 2 months) than the contrasting studies (2-3 weeks).
Motor function (Upper extremity)
Effective
1A
Six high quality RCTs (Wu et al., 2007a, Wu et al., 2007b; Abu Tariah et al., 2010; Khan et al., 2011;Smania et al., 2012; Batool et al., 2015) and 1 fair quality RCT (Wang et al., 2011) examined the effects of mCIMT on motor function in patients with stroke.
The first high quality RCT (Wu et al., 2007a) randomized patients between 3 weeks and 35 months since stroke to receive mCIMT or neurodevelopmental therapy (NDT). Patients in the mCIMT group received intensive functional training of the affected UE for 2 hours/day and wore a restraining mitt on the less-affected UE 6 hours/day. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the Fugl-Meyer Assessment (FMA). There was a significant between-group difference in motor function at post-treatment, in favour of mCIMT compared to NDT.
The second high quality RCT (Wu et al., 2007b) randomized patients between 3 weeks and 35 months since stroke to receive mCIMT or NDT. Patients in the mCIMT group received intensive functional training of the affected UE for 2 hours/day and wore a restraining mitt on the less-affected UE 6 hours/day. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the FMA. There was a significant between-group difference in motor function at post-treatment, in favour of mCIMT compared to NDT.
The third high quality RCT (Abu Tariah et al., 2010) randomized patients with subacute or chronic stroke to receive mCIMT or NDT for 2 hours/day, 7 days/week. Upper extremity motor function was measured at baseline, 2 months (post-treatment) and 6-month follow-up using the Wolf Motor Function Test – Performance Time, Functional Ability (WMFT-PT, WMFT-FA) and the Fugl-Meyer Assessment (FMA). There was a significant between-group difference in WMFT–FA scores at post-treatment in favour of mCIMT compared to NDT. No significant difference in WMFT–PT or FMA was seen between groups at either time point.
The fourth high quality RCT (Khan et al., 2011) randomized patients with subacute to chronic stroke to receive mCIMT, conventional neurological therapy or therapeutic climbing. The mCIMT group received physiotherapy and occupational therapy for 5 hours/week while wearing a mitt, and an additional 5 hours/week of self-training of repetitive task-oriented activities. Upper extremity motor function was measured at baseline, 5 weeks (post-treatment) and 6-month follow-up using the WMFT-PT and WMFT-FA. There was a significant between-group difference in one measure of upper extremity motor function (WMFT – Time) at post-treatment (approximately 32 days) and at 6-month follow-up, in favour of mCIMT compared to therapeutic climbing. There were no significant differences in WMFT–FA scores at either time point.
The fifth high quality RCT (Smania et al., 2012) randomized patients with subacute to chronic stroke to receive modified constraint-induced movement therapy or conventional rehabilitation. The mCIMT group wore a splint on the unaffected arm for 12 or more hours/day and received rehabilitation two hours/weekday. Upper extremity motor function was measured at baseline, 2 weeks (post-treatment) and 3-month follow-up using the WMFT-PT and WMFT-FA. There was a significant between-group difference in WMFT-FA scores at post-treatment and follow-up, in favour of mCIMT compared to conventional rehabilitation. There was no significant between-group difference in WMFT-PT scores at either time point.
The sixth high quality RCT (Batool et al., 2015) randomized patients who were 2 weeks to 3 months post-stroke to receive mCIMT or a motor relearning program. The mCIMT group wore a mitt during 2-hour training sessions, 6 times/week. Upper extremity motor function was measured at baseline and 3 weeks (post-treatment) using the Motor Assessment Scale (MAS) – upper arm function, hand movements, advanced hand activities and total scores. There were significant between-group differences on all MAS domains at post-treatment, in favour of mCIMT compared to the motor relearning program.
The fair quality RCT (Wang et al., 2011) randomized patients with acute or subacute stroke to receive mCIMT, intensive conventional rehabilitation, or conventional rehabilitation. The mCIMT group received occupational therapy 3 hours/weekday and wore a resting hand splint 90% of the time. Upper extremity motor function was measured at baseline and at 2 weeks and 4 weeks (post-treatment) using the WMFT-PT and WMFT-FA. There were significant between-group differences in WMFT-FA scores at 2 weeks, and in WMFT-PT scores at 4 weeks, in favour of mCIMT compared to conventional rehabilitation. There were no significant differences between mCIMT and intensive conventional rehabilitation, or between intensive conventional rehabilitation and conventional rehabilitation.
Conclusion: There is strong evidence (level 1a) from 6 high quality RCTs and 1 fair quality RCT that mCIMT is more effective than control therapies (neurodevelopmental therapy, conventional rehabilitation, therapeutic climbing, motor relearning program) for improving upper extremity motor function in patients with stroke.
Note: One high quality RCT found no significant difference between mCIMT and conventional neurological therapy; one fair quality RCT found no significant difference between mCIMT and intensive conventional rehabilitation.
Range of motion
Not effective
1b
One high quality RCT (Khan et al., 2011) investigated the effect of mCIMT on upper extremity range of motion in patients with stroke. This high quality RCT randomized patients with subacute to chronic stroke to receive mCIMT, conventional neurological therapy, or therapeutic climbing. The mCIMT group received physiotherapy and occupational therapy for 5 hours/week while wearing a mitt, and an additional 5 hours/week of self-training of repetitive task-oriented activities. Upper extremity active range of motion (shoulder flexion) was measured using a goniometer at baseline, 5 weeks (post-treatment) and 6-month follow-up. There were no significant between-group differences in active range of motion on shoulder flexion at any time point.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is not more effective than comparison interventions (conventional neurological therapy, therapeutic climbing) for improving upper extremity range of motion in patients with stroke.
One high quality RCT (Khan et al., 2011) investigated the effect of mCIMT on shoulder pain among patients with stroke. This high quality RCT randomized patients with subacute to chronic stroke to receive conventional neurological therapy, modified CIMT or therapeutic climbing. The mCIMT group received physiotherapy and occupational therapy for 5 hours/week while wearing a mitt, and an additional 5 hours/week of self-training of repetitive task-oriented activities. Pain was measured at baseline, 5 weeks (post-treatment) and 6-month follow-up using the Chedoke McMaster Impairment Inventory. There were significant between-group differences in pain at post-treatment and follow-up, in favour of mCIMT compared to therapeutic climbing. There were significant between-group differences in pain at follow-up only in favour of mCIMT compared to conventional neurological therapy.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is more effective than comparison intervention (therapeutic climbing) for reducing shoulder pain in patients with stroke.
Note: mCIMT was also found to be more effective than conventional neurological therapy for minimizing shoulder pain long-term.
Spasticity (Upper extremity)
Not effective
1B
One high quality RCT (Smania et al., 2012) has examined the effect of mCIMT on spasticity following stroke. This high quality RCT randomized patients with subacute to chronic stroke to receive mCIMT or conventional rehabilitation. The mCIMT group received rehabilitation two hours/weekday and wore a splint on the unaffected arm for 12 or more hours/day. Spasticity was measured at baseline, 2 weeks (post-treatment) and 3-month follow-up using the Ashworth Scale. There were no significant between-group differences in upper extremity spasticity at post-treatment or follow-up.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is not more effective than conventional rehabilitation for improving spasticity following stroke.
Strength (Upper extremity)
Not effective
1B
One high quality RCT (Khan et al., 2011) investigated the effect of mCIMT on upper extremity strength in patients with stroke. This high quality RCT randomized patients with subacute to chronic stroke to receive conventional neurological therapy, modified CIMT or therapeutic climbing. Modified CIMT comprised group-based physiotherapy and occupational therapy for 5 hours/week while wearing a mitt, and an additional 5 hours/week of self-training of repetitive task-oriented activities. Upper extremity strength was measured at baseline, 5 weeks (post-treatment) and 6-month follow-up using the WMFT-Strength domain and a hand-held dynamometer. There were no significant between-group differences in isometric strength on shoulder or elbow flexion/extension at either time point.
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is not more effective than comparison interventions (conventional neurological therapy, therapeutic climbing) for improving upper extremity strength in patients with stroke.
Stroke outcomes
Effective
1B
One high quality RCT (Wu et al., 2007b) has examined the effect of mCIMT on stroke outcomes. This high quality RCT randomized patients between 3 weeks and 35 months since stroke to receive mCIMT or neurodevelopmental therapy (NDT). Stroke outcomes were measured at baseline and 3 weeks (post-treatment) using the Stroke Impact Scale (SIS). There were significant between-group differences in stroke outcomes (SIS strength, ADL/IADL and stroke recovery domains) at post-treatment, in favour of mCIMT compared to NDT. There were no significant between-group differences in other SIS domains (hand function, memory and thinking, emotion, communication, participation, mobility).
Conclusion: There is moderate evidence (level 1b) from 1 high quality RCT that mCIMT is more effective than control therapy (e.g. neurodevelopmental therapy) for improving some stroke outcomes.
References
Abo, M., Kakuda, W., Momosaki, R., Harashima, H., Kojima, M., Watanabe, S., Sato, T., Yokoi, A., Umemori, T., & Sasanuma, J. (2014). Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: the NEURO-VERIFY Study. International Journal of Stroke, 9(5), 607-12. http://www.ncbi.nlm.nih.gov/pubmed/24015934
Abu Tariah, H., Almalty, A-M., Sbeih, Z., & Al-Oraibi, S. (2010). Constraint induced movement therapy for stroke Abu Tariah, H., Almalty, A-M., Sbeih, Z., & Al-Oraibi, S. (2010). Constraint induced movement therapy for stroke survivors in Jordan: a home-based model. International Journal of Therapy and Rehabilitation, 17(12), 638-46.
Alberts, J.L., Butler, A.J., & Wolf, S.L. (2004). The effects of constraint-induced therapy on precision grip: A preliminary study. Neurorehabilitation and Neural Repair, 18(4), 250-8. http://www.ncbi.nlm.nih.gov/pubmed/15537995
Atteya, A-A.A. (2004). Effects of modified constraint induced therapy on upper limb function in sub-acute stroke patients. Neurosciences, 9(1), 24-9. http://www.ncbi.nlm.nih.gov/pubmed/23377299
Azab M, Al-Jarrah M, Nazzal M, Maayah M, Sammour MA, Jamous M. (2009). Effectiveness of constraint-induced movement therapy (CIMT) as home-based therapy on Barthel Index in patients with chronic stroke. Top Stroke Rehabil, 16(3), 207-211. http://www.ncbi.nlm.nih.gov/pubmed/19632965
Bang, D.H., Shin, W.S., & Choi, H.S. (2015). Effects of modified constraint-induced movement therapy combined with trunk restraint in chronic stroke: A double-blinded randomized controlled pilot trial. NeuroRehabilitation, 37(1), 131-7. http://www.ncbi.nlm.nih.gov/pubmed/26409698
Barzel A, Liepert J, Haevernick K, Eisele M, Ketels G, Rijntjes M, van den Bussche H. Comparison of two types of Constraint-Induced Movement Therapy in chronic stroke patients: A pilot study. Restor Neurol Neurosci. 2009;27(6):673-80. https://www.ncbi.nlm.nih.gov/pubmed/20042791
Barzel, A., Ketels, G., Stark, A., Tetzlaff, B., Daubmann, A., Wegscheider, K., van den Bussche, H., & Scherer, M. (2015). Home-based constraint-induced movement therapy for patients with upper limb dysfunction after stroke (HOMECIMT): a cluster-randomised, controlled trial. Lancet Neurology, 14(9), 893-902. http://www.ncbi.nlm.nih.gov/pubmed/26231624
Batool., S., Soomro, N., Amjad, F., & Fauz, R. (2015). To compare the effectiveness of constraint induced movement therapy versus motor relearning programme to improve motor function of hemiplegic upper extremity after stroke. Pakistan Journal of Medical Sciences, 31(5), 1167-71. http://www.ncbi.nlm.nih.gov/pubmed/26649007
Boake C., Noser E., Ro,T., Barabiuk S., Gaber M., Johnson R., Salmeron E.T., Tran T.M., Lai J.M., Taub E., Moye L.A., Grotta J.C. & Levin, H.S. (2007). Constraint-induced movement therapy during early stroke rehabilitation. Neurological Neural Repair, 21, 14-24. http://www.ncbi.nlm.nih.gov/pubmed/17172550
Brogårdh, C. & Lexell, J. (2010). A 1-year follow-up after shortened constraint-induced movement therapy with and without mitt poststroke. Archives of Physical Medicine and Rehabilitation, 91, 460-4. http://www.ncbi.nlm.nih.gov/pubmed/20298840
Brogårdh, C., & Sjülund, B.H. (2006). Constraint-induced movement therapy in patients with stroke: a pilot study on effects of small group training and of extended mitt use. Clinical Rehabilitation, 20, 218-27. http://www.ncbi.nlm.nih.gov/pubmed/16634340
Brogårdh, C., Sjülund, B.H., & Lexell, J. (2009a). What is the long-term benefit of constraint-induced movement therapy? A four-year follow-up. Clinical Rehabilitation, 23, 418-23. http://www.ncbi.nlm.nih.gov/pubmed/19349341
Brogårdh, C., Vestling M., & B.H.Sjölund. (2009b). Shortened constraint-induced movement therapy in subacute stroke – No effect of using a restraint: a randomized controlled study with independent observers. J Rehabil Med, 41, 231-236. http://www.ncbi.nlm.nih.gov/pubmed/19247541
Brunner IC, Skouen JS, Strand LI. Is modified constraint-induced movement therapy more effective than bimanual training in improving arm motor function in the subacute phase post stroke? A randomized controlled trial. Clin Rehabil. 2012 Dec;26(12):1078-86. https://www.ncbi.nlm.nih.gov/pubmed/22561098
Brunner, I.C., Skouen, J.S., & Strand, L.I. (2012). Is modified constraint-induced movement therapy more effective than bimanual training in improving upper arm motor function in the subacute phase post stroke? A randomized controlled trial. Clinical Rehabilitation, 26(12), 1078-86. http://www.ncbi.nlm.nih.gov/pubmed/22561098
Caimmi M., Carda S., Giovanzana C., Maini E.S., Sabatini A.M., Smania N. & Molteni F. (2008).Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil Neural Repair, 22, 31-39. http://www.ncbi.nlm.nih.gov/pubmed/17595381
Corbetta, D., Sirtori, V., Moja, L., & Gatti, R. (2010). Constraint-induced movement therapy in stroke patients: systematic review and meta-analysis. European Journal of Physical Rehabilitation and Medicine, 46, 537-44. http://www.ncbi.nlm.nih.gov/pubmed/21224785
Dahl A.E., Askim T., Stock R., Langørgen E., Lydersen S. & Indredavik B., (2008). Short- and long-term outcome of constraint-induced movement therapy after stroke: A randomized controlled feasibility trial. Clinical Rehabilitation, 22, 436-447. http://www.ncbi.nlm.nih.gov/pubmed/18441040
Dettmers C., Teske U., Hamzei F., Uswatte G., Taub E., & Weiller, C. (2005). Distributed form of constraint-induced movement therapy improves functional outcome and quality of life after stroke. Arch Phys Med Rehabil, 86(2), 204-209. http://www.ncbi.nlm.nih.gov/pubmed/15706544
Dromerick A. W., Edwards D. F., & Hahn, M. (2000). Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke, 31(12), 2984-2988. http://www.ncbi.nlm.nih.gov/pubmed/11108760
Dromerick A.W, Lang C.E, Birkenmeier, R.L., Wagner J.M., Miller J.P., Videen T.O., Powers W.J., Wolf S.L., Edwards D.F. (2009) Very Early Constraint-Induced Movement during troke Rehabilitation (VECTORS): a single-center RCT. Neurology, 73(3), 195. http://www.ncbi.nlm.nih.gov/pubmed/19458319
El-Helow, M.R., Zamzam, M.L., Fathalla, M.M., El-Badawy, M.A., El Nahhas, N., El-Nabil, L.M., Awad, M.R., & Von Wild, K. (2015). Efficacy of modified constraint-induced movement therapy in acute stroke. European Journal of Physical and Rehabilitation Medicine, 51(4), 371-9. http://www.ncbi.nlm.nih.gov/pubmed/25030204
Hammer, A. & Lindmark, B. (2009a). Is forced use of the paretic upper limb beneficial? A randomized pilot study during sub-acute post-stroke recovery. Clinical Rehabilitation, 23, 424-33. http://www.ncbi.nlm.nih.gov/pubmed/19321522
Hammer, A. M. & Lindmark, B. (2009b). Effects of forced use on arm function in the sub-acute phase after stroke: A randomized, clinical pilot study. Physical Therapy, 89(6), 526-39. http://www.ncbi.nlm.nih.gov/pubmed/19372172
Hayner K, Gibson G, Giles GM. Comparison of constraint-induced movement therapy and bilateral treatment of equal intensity in people with chronic upper-extremity dysfunction after cerebrovascular accident. Am J Occup Ther. 2010 Jul-Aug;64(4):528-39. https://www.ncbi.nlm.nih.gov/pubmed/20825123
http://www.ncbi.nlm.nih.gov/pubmed/25229024
Huseyinsinoglu, B.E., Ozdincler, A.R., & Krespi, Y. (2012). Bobath Concept versus constraint-induced movement therapy to improve arm functional recovery in stroke patients: A randomized controlled trial. Clinical Rehabilitation, 26(8), 705-15. http://www.ncbi.nlm.nih.gov/pubmed/22257503
Khan, C.M., Oesch, P.R., Gamper, U.N., Kool, J.P., & Beer, S. (2011). Potential effectiveness of three different treatment approaches to improve minimal to moderate arm and hand function after stroke – a pilot randomized clinical trial. Clinical Rehabilitation, 25(11), 1032-41. http://www.ncbi.nlm.nih.gov/pubmed/21788267
Kim D.G., Cho Y.W., Hong J.H., Song J.C., Chung H.-A., Bai D.-S., Lee C.-H. & Jang S.H. (2008). Effect of constraint-induced movement therapy with modified opposition restriction orthosis in chronic hemiparetic patients with stroke. NeuroRehabilitation, 23(3), 239-244. http://www.ncbi.nlm.nih.gov/pubmed/18560140
Kunkel A, Kopp B, Müller G, Villringer K, Villringer A, Taub E, Flor H. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999 Jun;80(6):624-8. https://www.ncbi.nlm.nih.gov/pubmed/10378486
Lang, K.C., Thompson, P.A., & Wolf, S.L. (2013). The EXCITE Trial: Reacquiring upper-extremity task performance with early versus late delivery of constraint therapy. Neurorehabilitation and Neural Repair, 27(7), 654-63. http://www.ncbi.nlm.nih.gov/pubmed/23542218
Lin K., Chang Y., Wu C., Chen Y. (2009b). Effects of constraint-induced therapy versus bilateral arm training on motor performance, daily functions, and quality of life in stroke survivors. Neurorehabilitation and Neural Repair, 23(5), 441-448. http://www.ncbi.nlm.nih.gov/pubmed/19118130
Lin K., Wu C., Liu J.S. (2008). A randomized controlled trial of constraint-induced movement therapy after stroke. Acta Neurochir Suppl, 101, 61-64. http://www.ncbi.nlm.nih.gov/pubmed/18642635
Lin K.C., Wu C.Y., Liu J.S., Chen Y.T., Hsu C.J. (2009a). Constraint-Induced Therapy Versus Dose-Matched Control Intervention to Improve Motor Ability, Basic/Extended Daily Functions, and Quality of Life in Stroke. Neurorehabilitation and Neural Repair, 23(2), 160-165. http://www.ncbi.nlm.nih.gov/pubmed/18981188
Lin K.C., Wu C.Y., Wei T.H., Lee C.Y., & Liu J.S. (2007). Effects of modified constraint – induced movement therapy on reach to grasp movements and functional performance after chronic stroke: A randomized controlled study. Clinincal Rehabilitation, 21(12), 1075-1086. http://www.ncbi.nlm.nih.gov/pubmed/18042603
Lin, K-C., Chung, H-Y., Wu, C-Y., Liu, H-L., Hsieh, Y-W., Chen, I-H., Chen, C-L., Chuang, L-L., Liu, J-S. & Wai, Y-Y. (2010). Constraint-induced therapy versus control intervention in patients with stroke. A functional magnetic resonance imaging study. American Journal of Physical Medicine and Rehabilitation, 89, 177-85. http://www.ncbi.nlm.nih.gov/pubmed/20173425
Liu KP, Balderi K, Leung TL, Yue AS, Lam NC, Cheung JT, Fong SS, Sum CM, Bissett M, Rye R, Mok VC. A randomized controlled trial of self-regulated modified constraint-induced movement therapy in sub-acute stroke patients. Eur J Neurol. 2016 Aug;23(8):1351-60. doi: 10.1111/ene.13037. Epub 2016 May 19. https://www.ncbi.nlm.nih.gov/pubmed/27194393
Myint J., Yuen G., Kng C., Wong A., Chow K., & Li H. (2008). A study of constraint-induced movement therapy in subacute stroke patients in Hong Kong. Clinical Rehabilitation, 22, 112-124. http://www.ncbi.nlm.nih.gov/pubmed/18212033
Page S. J., Levine P., & Leonard, A. C. (2005). Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair, 19(1), 27-32. http://www.ncbi.nlm.nih.gov/pubmed/15673841
Page S. J., Sisto S., Johnston M. V., & Levine, P. (2002). Modified constraint-induced therapy after subacute stroke: A preliminary study. Neurorehabil Neural Repair, 16(3), 290-295. http://www.ncbi.nlm.nih.gov/pubmed/12234091
Page S. J., Sisto S., Levine P., & McGrath, R. E. (2004). Efficacy of modified constraint-induced movement therapy in chronic stroke: A single-blinded randomized controlled trial. Arch Phys Med Rehabil, 85(1), 14-18. http://www.ncbi.nlm.nih.gov/pubmed/14970962
Page S.J., Levine P., Leonard A., Szaflarski J.P., & Kissela, B.M. (2008). Modified constraint-induced therapy in chronic stroke: Results of a single-blinded randomized controlled trial. Physical Therapy, 88(3), 333-340. http://www.ncbi.nlm.nih.gov/pubmed/18174447
Page, S.J., Sisto, SA., Levine, P., Johnston, M.V., & Hughes, M. (2001). Modified constraint induced therapy: a randomized feasibility and efficacy study. Journal of Rehabilitation Research and Development, 38(5), 583-90. http://www.ncbi.nlm.nih.gov/pubmed/11732835
Ploughman M., & Corbett, D. (2004). Can forced-use therapy be clinically applied after stroke? An exploratory randomized controlled trial. Arch Phys Med Rehabil, 85(9), 1417-1423. http://www.ncbi.nlm.nih.gov/pubmed/15375810
Richards L, Senesac C, McGuirk T, Woodbury M, Howland D, Davis S, Patterson T. Response to intensive upper extremity therapy by individuals with ataxia from stroke. Top Stroke Rehabil. 2008 May-Jun;15(3):262-71. https://www.ncbi.nlm.nih.gov/pubmed/18647730
Sawaki L., Butler A.J., Leng X., Wassenaar P.A., Mohammad Y.M., Blanton S., Sathian K., Nichols-Larsen D.S., Wolf S.L., Good D.C. & Wittenberg G.F. (2008). Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabilitation and Neural Repair. 22(5), 505-513. http://www.ncbi.nlm.nih.gov/pubmed/18780885
Shi, Y. X., Tian, J. H., Yang, K. H., & Zhao, Y. (2011). Modified constraint-induced movement therapy versus traditional rehabilitation in patients with upper-extremity dysfunction after stroke: A systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation, 92, 972-982. http://www.ncbi.nlm.nih.gov/pubmed/21621674
Siebers, A. & Skargren, O.E. (2010). The effect of modified constraint-induced movement therapy on spasticity and motor function of the affected arm in patients with chronic stroke. Physiotherapy Canada, 62(4), 388-96. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2958081/
Sirtori, V., Corbetta, D., Moja, L., & Gatti, R. (2009). Constraint-induced movement therapy for upper extremities in stroke patients. Cochrane Database of Systematic Reviews, 4. Art. No.: DC004433. DOI: 10.1002/14651858.CD004433.pub2. http://www.ncbi.nlm.nih.gov/pubmed/19821326
Smania, N., Gandolfi, M., Paolucci, S., Iosa, M., Ianes, P., Recchia, S., Giovanzana, C., Molteni, F., Avesani, R., di Paolo, P., Zaccala, M., Agostini, M., Tassorelli, C., Fiaschi, A., Primon, D., Ceravolo, M.G., & Farina, S. (2012). Reduced-intensity modified constraint-induced movement therapy versis conventional therapy for upper extremity rehabilitation after stroke: A multicenter trial. Neurorehabilitation and Neural Repair, 26(9), 1035-45. http://www.ncbi.nlm.nih.gov/pubmed/22661278
Sterr A., Elbert T., Berthold I., Kolbel S., Rockstroh B., & Taub, E. (2002). Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: An exploratory study. Arch Phys Med Rehabil, 83(10), 1374-1377. http://www.ncbi.nlm.nih.gov/pubmed/12370871
Suputtitada A., Suwanwela N.C. & Tumvitee S. (2004). Effectiveness of constraint-induced movement therapy in chronic stroke patients. J Med Assoc Thai, 87(12), 1482-90. http://www.ncbi.nlm.nih.gov/pubmed/15822545
Taub E., Miller N. E., Novack T. A., Cook E. W., 3rd, Fleming W. C., Nepomuceno C. S., et al. (1993). Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil, 74(4), 347-354. http://www.ncbi.nlm.nih.gov/pubmed/8466415
Taub, E., Uswatte, G., King, D.K., Morris, D., Crago, J.E., & Chatterjee, A. (2006). A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke, 37, 1045-9. http://www.ncbi.nlm.nih.gov/pubmed/16514097
Thrane, G., Askim, T., Stock, R., Indredavik, B., Gjone, R., Erichsen, A., & Anke, A. (2015). Efficacy of constraint-induced movement therapy in early stroke rehabilitation: A randomized controlled multisite trial. Neurorehabilitation and Neural Repair, 29(6), 517-25. http://www.ncbi.nlm.nih.gov/pubmed/25398726
Treger, I., Aidinof, L., Lehrer, H., & Kalichman, L. (2012). Modified constraint-induced movement therapy improved upper limb function in subacute poststroke patients: A small-scale clinical trial. Topics in Stroke Rehabilitation, 19(4), 287-93. http://www.ncbi.nlm.nih.gov/pubmed/22750958
Underwood, J., Clark, P.C., Blanton, S., Aycock, D.M., & Wolf, S.L. (2006). Pain, fatigue and intensity of practice in people with stroke who are receiving constraint-induced movement therapy. Physical Therapy, 86(9), 1241-50. http://www.ncbi.nlm.nih.gov/pubmed/16959672
van der Lee J. H., Wagenaar R. C., Lankhorst G. J., Vogelaar T. W., Deville W. L., & Bouter, L. M. (1999). Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke, 30(11), 2369-2375. http://www.ncbi.nlm.nih.gov/pubmed/10548673
Wang, Q., Zhao, J-l., Zhu, Q-x., Li, J., & Meng, P-p. (2011). Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. Journal of Rehabilitation Medicine, 43, 619-25. http://www.ncbi.nlm.nih.gov/pubmed/21603848
Wittenberg G. F., Chen R., Ishii K., Bushara K. O., Taub E., Gerber L.H., Hallett M., Cohen L.G. (2003). Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair, 17(1), 48-57. http://www.ncbi.nlm.nih.gov/pubmed/12645445
Wolf S.L., Winstein C.J., Miller J.P., Thompson P.A., Taub E., Uswatte G., Morris D., Blanton S., Nichols-Larsen D., Clark P.C. (2008). Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. The Lancet Neurology, 7(1), 33-40. http://www.ncbi.nlm.nih.gov/pubmed/18077218
Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D (2006). Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA; 296 (17): 2095-2104. http://www.ncbi.nlm.nih.gov/pubmed/17077374
Wolf, S.L., Thompson, P.A., Winstein, C.J., Miller, J.P., Blanton, S.R., Nichols-Larsen, D.S., Morris, D.M., Uswatte, G., Taub, E., Light, K.E., Sawaki, L. (2010). The EXCITE stroke trial: Comparing early and delayed constraint-induced movement therapy. Stroke, 41, 2309-15. http://www.ncbi.nlm.nih.gov/pubmed/20814005
Wu C.Y, Chen C.L., Tang S.F., Lin K.C., & Huang Y.Y. (2007a). Kinematic and clinical analysis of upper-extremity movement after constraint-induced movement therapy in patients with stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 88, 964-970. http://www.ncbi.nlm.nih.gov/pubmed/17678656
Wu C.Y., Chen C.L., Tsai W.C., Lin K.C., Chou, S.H. (2007b). A randomized controlled trial of modified constraint-induced movement therapy for elderly stroke survivors: Changes in motor impairment, daily functioning, and quality of life. Arch Phys Med Rehabil, 88, 273-278. http://www.ncbi.nlm.nih.gov/pubmed/17321816
Wu C.Y., Lin K.C., Chen H.C., Chen I.H., Hong W.H. (2007c). Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: A kinematic study of motor control mechanisms. Neurorehabil Neural Repair, 21, 460. http://www.ncbi.nlm.nih.gov/pubmed/17601803
Wu, C., Chen, Y., Chen, H., Lin, K., & Yeh, I. (2012a). Pilot trial of distributed constraint-induced therapy with trunk restraint to improve poststroke reach to grasp and trunk kinematics. Neurorehabilitation and Neural Repair, 26(3), 247-55. http://www.ncbi.nlm.nih.gov/pubmed/21903975
Wu, C., Chen, Y., Lin, K., Chao, C., & Chen, Y. (2012b). Constraint-induced therapy with trunk restraint for improving functional outcomes and trunk-arm control after stroke: a randomized controlled trial. Physical Therapy, 92(4), 483-92. http://www.ncbi.nlm.nih.gov/pubmed/22228607
Wu, C-y., Chuang, L-l., Lin, K-c., Chen, H-c., & Tsay, P-k. (2011). Randomized trial of distributed constraint-induced therapy versus bilateral arm training for the rehabilitation of upper-limb motor control and function after stroke. Neurorehabilitation and Neural Repair, 25, 130-9. http://www.ncbi.nlm.nih.gov/pubmed/20947493
Wu, C-Y., Hsieh, Y-W., Lin, K-C., Chuang, L-L., Chang, Y-F., Liu, H-L., Chen, C-L., Lin, K-W., & Wai, Y-Y. (2010). Brain reorganization after bilateral arm training and distributed constraint-induced therapy in stroke patients: A preliminary functional magnetic resonance imaging study. Chang Gung Medical Journal, 33, 628-38. http://www.ncbi.nlm.nih.gov/pubmed/21199608
Yoon, J.A., Koo, B.I., Shin, M.J., Shin, Y.B., Ko, H.Y., & Shin, Y.I. (2014). Effect of constraint-induced movement therapy and mirror therapy for patients with subacute stroke. Annals of Rehabilitation Medicine, 38(4), 458-66
Excluded Studies
Bang, D.H., Shin, W.S., & Choi, H.S. (2015) Effects of modified constraint-induced movement therapy combined with trunk restraint in chronic stroke: A double-blinded randomized controlled pilot trial. NeuroRehabilitation, 37(1), 131-7.
Reason for exclusion: Both groups received mCIMT.
Fuzaro, A.C., Guerreiro, C.T., Galetti, F.C., Juca, R.B.V.M., & de Araujo, J.E. (2012). Modified constraint-induced movement therapy and modified forced-use therapy for stroke patients are both effective to promote balance and gait improvements. Revista Brasileira de Fisioterapia [Brazilian Journal of Physical Therapy], 16(2), 157-65.
Reason for exclusion: Outcomes pertained to balance, gait and lower extremity function.
Gauthier, L.V., Taub, E., Perkins, C., Ortmann, M., Mark, V.W., & Uswatte, G. (2008). Remodeling the brain: Plastic structural brain changes produced by different motor therapies after stroke. Stroke, 39, 1520-5.
Reason for exclusion: Both groups received some form of mCIMT.
Krawczyk, M., Sidaway, M., Radwariska, A., Zaborska, J., Ujma, R., & Czlonkowska, A. (2012). Effects of sling and voluntary constraint during constraint-induced movement therapy for the arm after stroke: a randomized, prospective, single-centre, blinded observer rated study. Clinical Rehabilitation, 26(11), 990-8.
Reason for exclusion: Both groups received some form of constraint therapy.
Lima, R.C.M., Michaelsen, S.M., Nascimento, L.R., Polese, J.C., Pereira, N.D., & Teixeira-Salmela, L.F. (2014). Addition of trunk restraint to home-based modified constraint-induced movement therapy does not bring additional benefits in chronic stroke individuals with mild and moderate upper limb impairments: a pilot randomized controlled trial. NeuroRehabilitation, 35, 391-404.
Reason for exclusion: Both groups received mCIMT.